Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/012
REVIEW ARTICLE OPEN ACCESS
Outcome of Carotid Endarterectomy after Regional Anesthesia versus General Anesthesia A Retrospective Study Using Two Independent Databases
Jiabin Liu , MD, PhD, Hector Martinez-Wilson, MD, PhD, Mark D. Neuman, MD, MSCE, Nabil Elkassabany, MD, MSCE, Edward Andrew Ochroch, MD, MSCE
Department of Anesthesiology and Critical Care, the Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA 19104
Jiabin Liu, MD, PhD, Department of Anesthesiology and Critical Care, the Perelman School of Medicine, University of Pennsylvania, 3400 Spruce Street, Philadelphia, PA, USA 19104, Phone: 215-573-8239, Email: Jiabin.liu@uphs.upenn.edu
Citation: Jiabin Liu, Hector Martinez-Wilson, Mark D. Neuman, Nabil Elkassabany, Edward Andrew Ochroch. Outcome of Carotid Endarterectomy after Regional Anesthesia versus General Anesthesia A Retrospective Study Using Two Independent Database Trans Periop & Pain Med 2014, 1(2):*****
Abstract
Background
Carotid endarterectomy (CEA) is effective in reducing stroke risk in selected patient groups. The ideal anesthetic technique remains controversial in light of literature between general anesthesia (GA) and regional anesthesia (RA) for CEA.
Methods
We studied the NSQIP data from 2005 to 2012. There were 32, 718 patients receiving general anesthesia (GA) and 5, 384 patients receiving regional anesthesia, local anesthesia, or monitored anesthesia care (RA). The outcome measurements of 30 days postoperative complications were death, stroke, coma, unplanned intubation, on ventilator > 48 hours, cardiac arrest, and myocardial infarction. We next studied NY-SID data from 2007 to 2011. There were 13, 913 patients receiving GA and 3, 145 patients receiving RA. The outcome measurements by discharge time were death, stroke, paraplegia, new neurological disorder, aspiration, respiratory failure, pulmonary resuscitation procedure (include intubation), cardiac arrest, cardiac resuscitation procedure, myocardial infarction, and congestive heart failure. All analyses were risk adjusted with propensity score matching algorithm.
Results
There were significant differences in incidences of un-expected intubation (1.21% vs. 0.55%, P = 0.001), and myocardial infarction (0.80% vs. 0.35%, P = 0.039) between GA and RA respectively in NSQIP data. GA group had significant higher incidences of aspiration (0.61% vs. 0.19%, P = 0.014), and pulmonary resuscitation procedure (including intubation) (1.02% vs. 0.54%, P = 0.044) than RA group in NY-SID data.
Conclusions
In comparison to GA, patients receiving RA had significant lower risks of postoperative unplanned intubation and/or pulmonary resuscitation procedure after carotid endarterectomy.
Keywords
Carotid Endarterectomy; Regional Anesthesia; Outcome; NSQIP; NY-SID
Introduction
Carotid endarterectomy (CEA) is effective in reducing stroke risk in selected patient groups. CEA is commonly performed under general anesthesia (GA), regional anesthesia, local anesthesia, or monitored anesthesia care. Based on the typical intraoperative care paradigms, we chose to define regional anesthesia (RA) to include any of the above local anesthetic based anesthesia practice, including regional anesthesia, local anesthesia, and monitored anesthesia care. The choice of anesthesia is largely based on patient factors, surgeon's preference, and the culture of the institution. The ideal anesthetic technique remains controversial as multiple small studies produced conflicting results regarding the association of GA versus RA with mortality [1-7], stroke [2-8], hemodynamic homeostasis [1,3-5], and cardiac morbidity [3,6,7].
The GALA (general anesthesia versus local anesthesia for carotid surgery) study was the only large randomized controlled clinical trial with 3526 patients, and it concluded that there was no difference in incidences of death, stroke, or myocardial infarction between GA and RA [9]. While GALA study provided the most convincing comparisons between GA and RA, it has its limitation. The GALA study reported that 65% of the patients were ASA I or ASA II [9], while other study revealed ~90% of patients undergoing CEA were ASA III or IV [10].
The American College of Surgeons National Surgical Quality Improvement Program (NSQIP) is a nationally validated outcome-based program to measure surgical outcomes. It contains 140 variables, including patient demographic information, preoperative comorbidities, intraoperative variables, and 30-day postoperative complications. A retrospective study by Schechter and colleagues on the NSQIP data from 2005 to 2009 looking at the composite risks of stroke, myocardial infarction, and death did not show significant patient outcome differences between GA and RA groups (2.8% versus 3.6%) undergoing CEA [11]. However, Schechter et al. reported significant differences in secondary complications between GA and RA (4.1% versus 2.9%) without detail information on the nature of these differences [11]. Leichtle et al. studied the same NSQIP data from 2005 to 2009 with a propensity matching strategy, and concluded that GA was associated with higher incidence of myocardial infarction (odds ratio 5.41), while no differences were reported for mortality and stroke risks [10].
With the release of NSQIP data from 2010-2012 we proposed to take advantage of the much larger dataset to study low incidence clinically relevant postoperative complications during CEA. We hypothesized that there are no differences on 30-day postoperative central nervous, pulmonary, and cardiovascular system complications between GA and RA patients.
New York State Inpatient Database is another independent database publically available via the US Agency for Healthcare Research and Quality's (AHRQ) Health Care Utilization Project (HCUP). The database contains information on patient demographic information, International Classification of Diseases-9-Clinical Modification (ICD-9-CM) code for diagnoses, ICD-9-CM code for procedures, anesthesia type, and discharge status. There was no previous study on outcome differences between GA and RA among CEA patients in the NY-SID data. Hereby we propose to utilize the NY-SID data as a replication set to further test our hypothesis.
Materials and Methods
Data source
This study was exempted by the institutional review board (the University of Pennsylvania, Philadelphia, Pennsylvania, USA 19104).
NSQIP Data: We acquired the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database from 2005 to 2012 [http://site.acsnsqip.org]. NSQIP members prospectively submit data on 140 variables, which are validated via strict standardized protocol. The data include demographic information, pre-existing comorbidities, intraoperative variables, and postoperative complications for 30 days after the surgery. The full list of information collected is available at NSQIP [http://site.acsnsqip.org/participant-use-data-file/]. The NSQIP participant user data files included 152,490 subjects in 2005 and 2006, 211,407 subjects in 2007, 271,368 subjects in 2008, 336,190 subjects in 2009, 363,431 subjects in 2010, 442,149 subjects in 2011, and 543,885 subjects in 2012.
NY-SID Data: We acquired the US Agency for Healthcare Research and Quality's (AHRQ) Healthcare Cost and Utilization Project (HCUP) New York State Inpatient Database (NY-SID) from 2007 to 2011 (http://www.hcup-us.ahrq.gov). NY-SID includes the collection of all encounter-level information in the state of New York. The data include demographic information, anesthesia type, ICD-9-CM diagnosis code, ICD-9-CM procedure code, AHRQ comorbidity measures of various organs/systems, and discharge status. The full list of information collected is available online (http://www.hcup-us.ahrq.gov/db/state/siddist/sid_multivar.jsp, last accessed August 15, 2014). The NY-SID data included 2,608,615 subjects in 2007, 2,629,383 subjects in 2008, 2,661,905 subjects in 2009, 2,612,610 subjects in 2010, and 2,578,680 subjects in 2011.
Study Sample Definition
NSQIP Study Sample: To define our study cohort in NSQIP data, we included patients with the Current Procedural Terminology (CPT) code for carotid endarterectomy as their principal procedure (CPT code 35301). There were total of 54, 450 entries with the listed CPT code as principal procedure. We first removed patients with ICD-9-CM diagnosis other than carotid occlusion and stenosis (ICD-9 433.1, 433.10, or 433.11). We next excluded patients with other significant concurrent procedures as defined by a relevant concurrent CPT code, which could have significantly effects on the choice of anesthesia and postoperative complication rates. (e.g. combined CEA and CABG) We elected to apply work relative value unit > 2.11 as the cutoff criteria to eliminate patients with significant concurrent procedures, while maintaining patients with relevant benign procedures that were relevant to carotid endarterectomy (such as angiography, ultrasonography, arterial cannulation, etc.). We then removed entries with more than five missing comorbidity data points. Next, we excluded patients who received anesthesia type other than general, local, regional, or monitored anesthesia care. We also excluded patients with ASA classification 5 (moribund). Last, we excluded patients who had prior operations within 30 days, pneumonia, ventilator dependence, systemic inflammatory response syndrome (SIRS), sepsis, septic shock, or contaminated/infected/dirty wound classification preoperatively. A diagram illustrating the defining process is summarized in figure 1.
NY-SID Study Sample: To define our study cohort in NY-SID data, we included patients with the primary diagnosis of carotid occlusion and stenosis with or without cerebral infarction (ICD-9-CM 433.10 or 433.11). There were total of 25, 336 entries. We first removed patients without ICD-9-CM procedure code (3812) in the first three listed procedures. Next, we excluded patients who received anesthesia type other than general, local, or regional anesthesia. Last, we excluded patients who had pneumonia or were ventilator dependent preoperatively. A diagram illustrating the defining process is summarized in figure 2.
Exposure Variable
The NSQIP Participant User Data File coded anesthesia types into the following categories: general, local, regional, monitored anesthesia care, spinal, epidural, other, none, or unknown. In cases where general anesthesia was used concurrently with other type(s) of anesthesia, patients were coded as receiving general anesthesia. For the present study, we grouped patients receiving local, regional, or monitored anesthesia care together in a single category as regional anesthesia.
The NY-SID codes method of anesthesia types into the following categories: Local, general, regional, other, none, or unknown. In cases where general anesthesia was used concurrently with other type(s) of anesthesia, patients were coded as receiving general anesthesia. For the present study, we grouped patients receiving local and regional anesthesia together in a single category as regional anesthesia.
Study Variables
The NSQIP dataset contains demographic information (age, gender, height, weight, race), type of anesthesia, American Society of Anesthesiologists (ASA) Physical Status Classification, level of functional dependence prior to surgery in activities of daily living, wound classification, and comorbidities. For this study, we created variables corresponding to the individual system or organ: Severe chronic obstructive pulmonary disease (COPD), congestive heart failure, coronary artery disease (defined as history of myocardial infarction, prior percutaneous coronary intervention, previous cardiac surgery, or history of angina in one month before surgery), peripheral vascular disease (defined as history of revascularization/amputation for peripheral vascular disease, or rest pain/gangrene), hypertension requiring medications, diabetes mellitus with or without insulin treatment, end stage liver disease (defined as presence of ascites, or esophageal varices), kidney failure (defined as acute renal failure, or currently on dialysis), central nervous system (CNS) disease (defined as impaired sensorium, coma>24 hours, history of transient ischemic attack, cerebrovascular accident/stroke with or without neurological deficit, or tumor involving CNS), spinal cord injury (defined as hemiplegia, paraplegia, or quadriplegia), and active malignancy (defined as disseminated cancer, chemotherapy, or radiotherapy for malignancy).
The NY-SID dataset contains demographic information (age, gender, race), type of anesthesia, ICD-9-CM diagnosis code, diagnosis present on admission indicator, ICD-9-CM procedure code, and AHRQ HCUP comorbidity measures. The comorbidity measures include congestive heart failure, pulmonary circulation disorders, peripheral vascular disease, chronic pulmonary disease, diabetes, liver disease, renal failure, central nervous system disease, malignancy, and etc. The full list is available at http://www.hcup-us.ahrq.gov/db/state/sasddist/sasd_multivar.jsp; last accessed on Aug 15, 2014. The definition of each comorbidity by ICD-9-CM code is available at http://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/Table2-FY12-V3_7.pdf, last accessed on Aug 15, 2014.
Study Variables
We obtained data on six postoperative complications within NSQIP database. These included stroke/CVA, coma > 24 hours, unplanned intubation, on ventilator > 48 hours, cardiac arrest requiring CPR, and myocardial infarction. All six variables were defined as either diagnosed by surgeon or attending physician, or on the basis of pre-defined clinical and laboratory criteria as specified at NSQIP website.
We were able to obtain data on 10 postoperative complications within NY-SID database. These included stroke, paralysis, new neurological disorder, aspiration, respiratory failure, pulmonary resuscitation procedure (including reintubation and extended ventilator support), cardiac arrest, cardiac resuscitation procedure, myocardial infarction, and congestive heart failure. Each variable is identified via ICD-9-CM code algorithms as previously described [12-15], with additional ICD-9-CM coding algorithms on paralysis and new neurological disorder at HCUP website(http://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/Table2-FY12-V3_7.pdf), last accessed on Aug 15, 2014).
Statistical Analysis
A propensity score was calculated with logistic regression modeling that included all study variables. Variables for the NSQIP matching algorithm include age, gender, BMI, race, ASA Status, level of functional status, and all pre-existing comorbidities. Variables for the NY-SID pairing include age, gender, race, and AHRQ comorbidity measures. The propensity score represented the probability of receiving RA for each patient in the range of 0 to 1. The propensity scores were then applied to create two matched groups of GA and RA with the caliper distance of 0.005 without replicates. The matched cohorts were then compared similarly as described above. All data analyses were executed in STATA 12.1 (StataCorp LP, College Station, TX, USA). Fisher's exact test and chi-square test were used for categorical data. Student T-test and Wilcoxon test were applied for interval data. Statistic significance was defined as P < 0.05. Our main focus was to compare individual central nervous, pulmonary, and cardiac system outcomes between GA and RA groups. To compensate the existing differences on patient characteristics and comorbidities between GA and RA groups, we conducted propensity score matching algorithm (described below) to generate two matching groups of GA and RA patients for further analysis.
Results
The cohort from the NSQIP database
We identified 54, 450 patients who underwent carotid endarterectomy via CPT code between 2005 and 2012 in the NSQIP database (Figure 1). We excluded 2973 patients with concurrent ICD-9 codes other than 433.1, 433.10, or 433.11 (Carotid artery occlusion and stenosis). We then removed 1432 entries with major concurrent procedures, and 10, 653 entries with more than 5 missing pre-existing comorbidity data fields. We next excluded 492 patients with types of anesthesia other than local, regional, monitored anesthesia care, or general anesthesia. We further eliminated patients with ASA classification 5 - moribund (n=10), unknown ASA classification (n=27), prior operations within 30 days (n=366), preoperative pneumonia (n=45), ventilator dependent (n=6), systemic inflammatory response syndrome (SIRS)/sepsis/septic shock (n=242), and contaminated/infected/dirty wound classification (n=102). The final cohort contained 38, 102 patients (Figure 1).
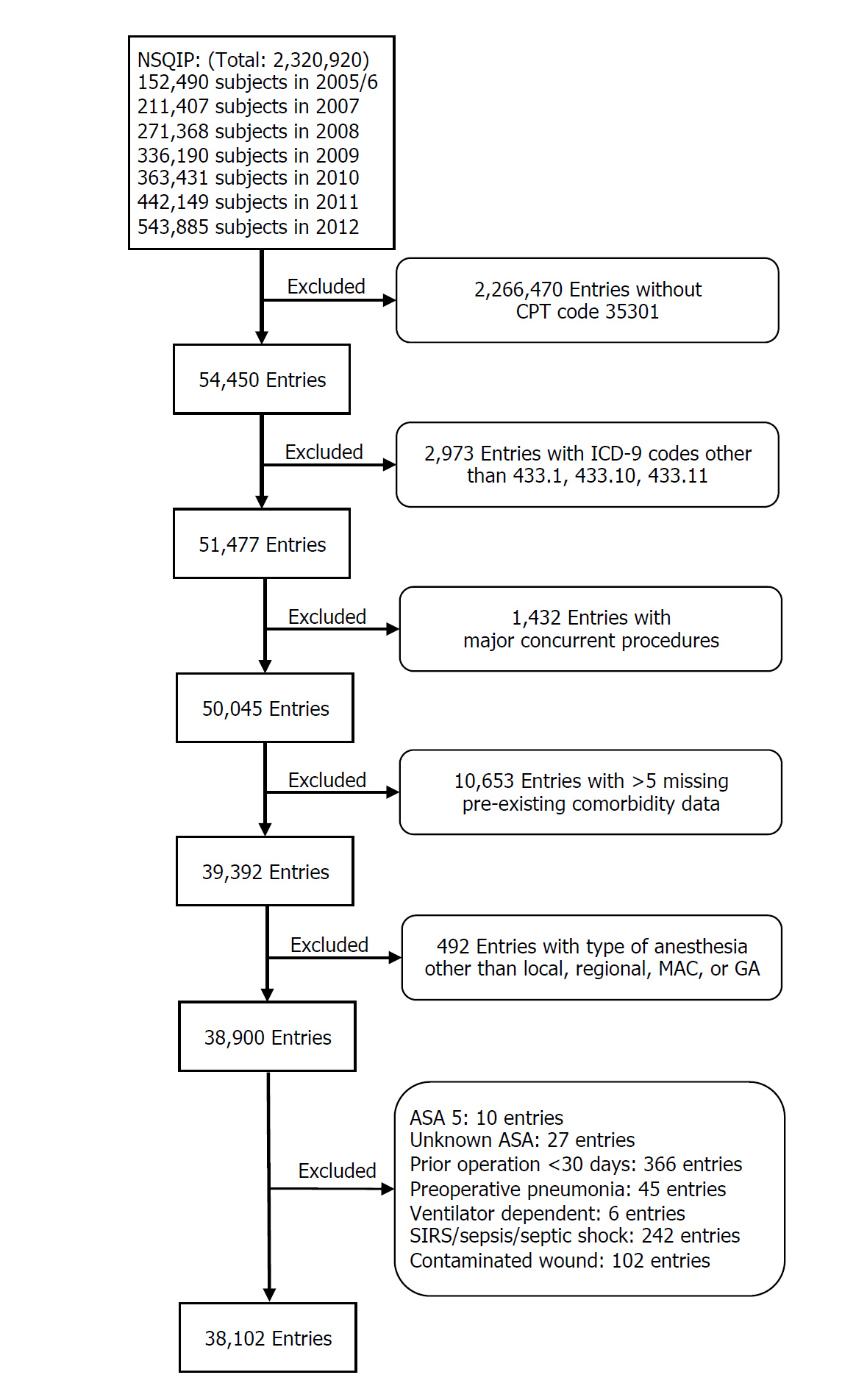
Figure 1: Creation of study sample with NSQIP database. CPT: Current Procedural Terminology. ICD-9: International Classification of Diseases-9. MAC: Monitored Anesthesia Care. GA: General Anesthesia.
There were 32, 718 (85.87%) GA, and 5384 (14.13%) RA subjects in the NSQIP dataset (Table 1). There were statistical, but probably not clinically, significant differences in age, gender, BMI, and race. The average hospitalization days were 2.47 vs. 2.09 days between GA and RA group (P < 0.0001).
Table 1: Categories of KOR agonists
| General Anesthesia | Regional Anesthesia | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| N/Mean | %/SD | N/Mean | %/SD | |||||
| Total Subjects (N) | 32718 | ± | 85.87% | 5384 | ± | 14.13% | ||
| Age | 70.94 | ± | 9.51 | 72.15 | ± | 9.32 | < 0.0001 | |
| Gender: | Male | 19271 | 59.00% | 3243 | 60.45% | 0.046 | ||
| Female | 13393 | 41.00% | 2122 | 39.55% | ||||
| Unknown | 54 | 19 | ||||||
| BMI | 28.13 | ± | 6.63 | 27.81 | ± | 6.16 | 0.0009 | |
| Race | White | 27572 | 90.60% | 4502 | 92.24% | < 0.001 | ||
| Black | 1344 | 4.42% | 147 | 3.01% | ||||
| Hispanic | 988 | 3.25% | 151 | 3.09% | ||||
| Others | 529 | 1.74% | 81 | 1.66% | ||||
| Unknown | 2285 | 503 | ||||||
| LOS | 2.47 | ± | 5.31 | 2.09 | ± | 6.06 | < 0.0001 | |
All values were reported as mean ± SD, or percentage.
The unit of measure for BMI is kilogram/meter2.
The GA group had higher prevalence of ASA class III and IV patients (91.14% vs 90.04%), COPD (10.51% vs 9.51%), diabetes on insulin (9.69% vs 8.64%), and central nervous system disease (43.33% vs 41.18%) than RA group (Table 2). The RA group had more patients with hypertension requiring medication (86.53% vs 85.20%), and spinal cord injury (0.54% vs 0.35%) than GA group. All subjects were then fitted with the propensity score matching analysis, and a subgroup of matched patients (n=4880 per group) from the total cohort was generated for further comparison. Analysis of preoperative demographic information and pre-existing comorbidities indicated covariate balance between GA and RA group (Table 3 and Table 4).
Table 2: NSQIP Prevalence of Pre-existing Comorbidities.
| Comorbidity | General Anesthesia | Regional Anesthesia | P-value | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| ASA classification | 4-Life Threat | 4201 | 12.84 | 568 | 10.55 | < 0.001 |
| 3-Severe Disturbance | 25618 | 78.30 | 4280 | 79.49 | ||
| 2-Mild Disturbance | 2850 | 8.71 | 533 | 9.90 | ||
| 1-No Disturbance | 49 | 0.15 | 3 | 0.06 | ||
| Functional health status Prior to Surgery | Dependent | 97 | 0.30 | 10 | 0.19 | 0.388 |
| Partially Dependent | 1375 | 4.21 | 225 | 4.18 | ||
| Independent | 31221 | 95.50 | 5149 | 95.64 | ||
| CHF in 30 days before surgery | 258 | 0.79 | 41 | 0.76 | 0.934 | |
| Coronary Artery Disease | 11785 | 36.02 | 1964 | 36.48 | 0.520 | |
| Peripheral Vascular Disease | 3257 | 10.01 | 509 | 9.45 | 0.210 | |
| HTN requiring medication | 27877 | 85.20 | 4659 | 86.53 | 0.010 | |
| Dyspnea | At Rest | 346 | 1.06 | 52 | 0.97 | 0.836 |
| Moderate Exertion | 5596 | 17.10 | 927 | 17.22 | ||
| History of severe COPD | 3439 | 10.51 | 512 | 9.51 | 0.026 | |
| End Stage Liver Disease | 26 | 0.08 | 5 | 0.09 | 0.795 | |
| Renal Failure | 376 | 1.15 | 49 | 0.91 | 0.141 | |
| Diabetes Mellitus | Insulin | 3172 | 9.69 | 465 | 8.64 | 0.047 |
| Oral/Non-insulin | 6062 | 18.53 | 1005 | 18.67 | ||
| Central Nervous System Disease | 14178 | 43.33 | 2217 | 41.18 | 0.003 | |
| Spinal Cord Injury | 115 | 0.35 | 29 | 0.54 | 0.042 | |
| Disseminated cancer, Chemotherapy/Radiotherapy | 148 | 0.45 | 27 | 0.50 | 0.587 | |
| Bleeding disorders | 6723 | 20.55 | 1083 | 20.12 | 0.477 | |
ASA: American Society of Anesthesiologists; CHF: Congestive heart failure; HTN: Hypertension; COPD: Chronic Obstructive Pulmonary Disease.
Table 3: NSQIP Patient Demographic Information Summary by Anesthesia Type in the Propensity Score Matched Sub-groups (N=4880 per group).
| General Anesthesia | Regional Anesthesia | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| N/Mean | %/SD | N/Mean | %/SD | |||||
| Age | 72.10 | ± | 9.21 | 72.16 | ± | 9.36 | 0.7462 | |
| Gender | Male | 3024 | 61.97% | 2956 | 60.57% | 0.164 | ||
| Female | 1856 | 38.03% | 1924 | 39.43% | ||||
| BMI | 27.86 | ± | 6.12 | 27.84 | ± | 6.16 | 0.8542 | |
| Race | White | 4502 | 92.25% | 4501 | 92.23% | 0.253 | ||
| Black | 144 | 2.95% | 147 | 3.01% | ||||
| Hispanic | 131 | 2.68% | 151 | 3.09% | ||||
| Others | 103 | 2.11% | 81 | 1.66% | ||||
All values were reported as mean ± SD, or percentage. The unit of measure for BMI is kilogram/meter2. "Other race" included patients listed as Native Hawaiian or Pacific Islander, Asian or Pacific Islander, Asian, and American Indian or Alaska Native in the NSQIP database.
Table 4: NSQIP Prevalence of Pre-existing Comorbidities in the Propensity Score Matched Sub-groups (N=4880 per group).
| Comorbidity | General Anesthesia | Regional Anesthesia | P-value | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | ||||
| ASA classification | 4-Life Threat | 520 | 10.66 | 528 | 10.82 | 0.381 | |
| 3-Severe Disturbance | 3927 | 80.47 | 3877 | 79.45 | |||
| 2-Mild Disturbance | 432 | 8.85 | 472 | 9.67 | |||
| 1-No Disturbance | 1 | 0.02 | 3 | 0.06 | |||
| Functional health status Prior to Surgery | Dependent | 7 | 0.14 | 7 | 0.14 | 0.975 | |
| Partially Dependent | 201 | 4.12 | 205 | 4.20 | |||
| Independent | 4672 | 95.74 | 4668 | 95.66 | |||
| CHF in 30 days before surgery | 34 | 0.70 | 38 | 0.78 | 0.723 | ||
| Coronary Artery Disease | 1766 | 36.19 | 1793 | 36.74 | 0.585 | ||
| Peripheral Vascular Disease | 449 | 9.20 | 458 | 9.39 | 0.780 | ||
| HTN requiring medication | 4241 | 86.91 | 4228 | 86.64 | 0.720 | ||
| Dyspnea | At Rest | 52 | 1.09 | 50 | 1.02 | 0.951 | |
| Moderate Exertion | 843 | 17.27 | 839 | 17.19 | |||
| History of severe COPD | 466 | 9.55 | 467 | 9.57 | 1 | ||
| End Stage Liver Disease | 9 | 0.18 | 5 | 0.10 | 0.424 | ||
| Renal Failure | 50 | 1.02 | 41 | 0.84 | 0.400 | ||
| Diabetes Mellitus | Insulin | 421 | 8.63 | 430 | 8.81 | 0.873 | |
| Oral/Non-insulin | 898 | 18.40 | 912 | 18.69 | |||
| Central Nervous System Disease | 1967 | 40.31 | 2015 | 41.29 | 0.333 | ||
| Spinal Cord Injury | 25 | 0.51 | 24 | 0.49 | 1 | ||
| Disseminated cancer, Chemotherapy/Radiotherapy | 30 | 0.61 | 26 | 0.53 | 0.688 | ||
| Bleeding disorders | 982 | 20.12 | 1000 | 20.49 | 0.669 | ||
ASA: American Society of Anesthesiologists; CHF: Congestive heart failure; HTN: Hypertension; COPD: Chronic Obstructive Pulmonary Disease.
Table 5 lists the incidence of central nervous, pulmonary, and cardiovascular system complications before and after propensity score matching within 30 days postoperatively in the NSQIP data. The overall 30 day mortality was 0.76% and 0.72% between GA and RA subjects respectively (P=0.906). The RA group had lower incidences of unplanned intubation after surgery (0.55% vs 1.21%, P=0.001), and myocardial infarction (0.45% vs 0.80%, P=0.039).
Table 5: NSQIP Incidences of 30-days Post-operative Complications before and after Propensity Score Matching
| Before Propensity Score Matching | After Propensity Score Matching | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GA(N=32718) | RA(N=5384) | GA(N=4880) | RA(N=4880) | |||||||
| Variable Label | N | % | N | % | P-value | N | % | N | % | P-value |
| Mortality | 238 | 0.73 | 37 | 0.69 | 0.795 | 37 | 0.76 | 35 | 0.72 | 0.906 |
| Stroke/CVA | 481 | 1.47 | 77 | 1.43 | 0.854 | 76 | 1.56 | 74 | 1.52 | 0.934 |
| Coma >24hours | 20 | 0.06 | 5 | 0.09 | 0.387 | 1 | 0.02 | 5 | 0.10 | 0.219 |
| Unplanned Intubation | 365 | 1.12 | 27 | 0.50 | < 0.001 | 59 | 1.21 | 27 | 0.55 | 0.001 |
| On Ventilator >48 Hours | 212 | 0.65 | 24 | 0.45 | 0.091 | 38 | 0.78 | 23 | 0.47 | 0.071 |
| Cardiac Arrest Requiring CPR | 84 | 0.26 | 10 | 0.19 | 0.377 | 9 | 0.18 | 10 | 0.20 | 1 |
| Myocardial Infarction | 257 | 0.79 | 25 | 0.46 | 0.010 | 39 | 0.80 | 22 | 0.45 | 0.039 |
GA: General anesthesia; RA: Regional anesthesia; CVA: Cerebrovascular accident; CPR: Cardiopulmonary resuscitation.
The cohort from the NY-SID data
We then set out to replicate these findings in the NSQIP data in the NY-SID data where we identified 25,336 patients with CEA listed within the first three procedures (Figure 2). We then removed 1863 patients without carotid occlusion and stenosis with or without cerebral infarction enlisted as primary diagnosis. Next, we excluded 6295 patients who received anesthesia type other than general, local, or regional anesthesia. Last, we excluded patients with preoperative pneumonia (n=4) and respiratory failure (n=116). The final cohort contained 17,058 subjects (Figure 2).

Figure 2: Creation of study sample with NY-SID database. ICD-9: International Classification of Diseases-9. ICD-9-CM: International Classification of Diseases-9 Clinical Modification. GA: General Anesthesia.
There were 13, 913 subjects in the GA group and 3,145 subjects in the RA group in the NY-SID patient's database (Table 6). The average length of hospitalization was 2.60 vs 2.05 days between GA and RA group (P < 0.001). Further analysis of the comorbidities indicated several differences (Table 7). The GA group had higher prevalence of patients with anemia (5.82% vs 4.29%), rheumatoid arthritis/collagen vascular disease (2.25% vs 1.53%), fluid/electrolyte disorder (5.11% vs 2.77%), and diabetes (32.16% vs 29.86%) than RA group. All subjects were then fitted with the propensity score matching analysis with NY-SID variables, and a subgroup of matched patients (n=3134 per group) from the total cohort was generated for further comparison. Analysis of preoperative demographic information and pre-existing comorbidities indicated the equality between GA and RA group (Table 8 and Table 9).
Table 6: NY-SID Patient Demographic Information Summary by Anesthesia Type.
| General Anesthesia | Regional Anesthesia | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| N/Mean | %/SD | N/Mean | %/SD | |||||
| Total Subjects (N) | 13913 | 81.56% | 3145 | 18.44% | ||||
| Age | 71.49 | ± | 9.43 | 71.71 | ± | 9.52 | 0.2433 | |
| Gender | Male | 8009 | 57.56% | 1889 | 60.06% | 0.010 | ||
| Female | 5904 | 42.44% | 1256 | 39.94% | ||||
| Race | White | 11942 | 86.52% | 2797 | 89.22% | < 0.001 | ||
| Black | 467 | 3.38% | 58 | 1.85% | ||||
| Hispanic | 755 | 5.47% | 107 | 3.41% | ||||
| Others | 638 | 4.62% | 173 | 5.52% | ||||
| LOS | 2.60 | ± | 4.91 | 2.05 | ± | 3.14 | < 0.001 | |
All values were reported as mean ± SD, or percentage. "Other race" included patients listed as Asian or Pacific Islander, Native American, or other in the NY-SID database. LOS: Length of Hospital Stay in days.
Table 7: NY-SID Patient Demographic Information Summary by Anesthesia Type in the Propensity Score Matched Sub-groups (N=3134 per group).
| General Anesthesia | Regional Anesthesia | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| N/Mean | %/SD | N/Mean | %/SD | |||||
| Age | 71.62 | ± | 9.39 | 71.70 | ± | 9.53 | 0.7224 | |
| Gender | Male | 1883 | 60.08% | 1883 | 60.08% | 1 | ||
| Female | 1251 | 39.92% | 151 | 39.92% | ||||
| Race | White | 2779 | 88.67% | 2796 | 89.22% | 0.916 | ||
| Black | 63 | 2.01% | 58 | 1.85% | ||||
| Hispanic | 112 | 3.7% | 107 | 3.41% | ||||
| Others | 180 | 5.4% | 173 | 5.52% | ||||
All values were reported as mean ± SD, or percentage. "Other race" included patients listed as Asian or Pacific Islander, Native American, and other in the NY-SID database.
Table 8: NY-SID Prevalence of Pre-existing Comorbidities in the Propensity Score Matched Sub-groups (N=3134 per group).
| Comorbidity | General Anesthesia | Regional Anesthesia | P-value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Alcohol | 49 | 1.56 | 48 | 1.53 | 1 |
| Deficiency Anemia | 117 | 3.73 | 135 | 4.31 | 0.274 |
| Rheumatoid Arthritis / Collagen Vascular Disease | 48 | 1.53 | 46 | 1.47 | 0.917 |
| Chronic Blood Loss Anemia | 9 | 0.29 | 10 | 0.32 | 1 |
| Congestive Heart Failure | 140 | 4.47 | 162 | 5.17 | 0.215 |
| Chronic Pulmonary Disease | 642 | 20.49 | 657 | 20.96 | 0.663 |
| Coagulopathy | 21 | 0.67 | 25 | 0.80 | 0.658 |
| Drug Abuse | 3 | 0.10 | 6 | 0.19 | 0.507 |
| Hypertension | 2563 | 81.78 | 2564 | 81.81 | 1 |
| Liver Disease | 25 | 0.80 | 29 | 0.93 | 0.682 |
| Fluid / Electrolyte Disorders | 73 | 2.33 | 87 | 2.78 | 0.298 |
| Obesity | 185 | 5.90 | 196 | 6.25 | 0.597 |
| Paralysis | 0 | 0 | 4 | 0.13 | 0.125 |
| Peripheral Vascular Disorders | 560 | 17.87 | 574 | 18.32 | 0.670 |
| Pulmonary Circulation Disorders | 32 | 1.02 | 34 | 1.08 | 0.902 |
| Renal Failure | 219 | 6.99 | 238 | 7.59 | 0.382 |
| Valvular Disease | 231 | 7.37 | 246 | 7.85 | 0.505 |
| Weight Loss | 4 | 0.13 | 8 | 0.26 | 0.387 |
| Mental Disorder | 199 | 6.35 | 213 | 6.80 | 0.508 |
| Cancer | 43 | 1.37 | 49 | 1.56 | 0.600 |
| Diabetes | 922 | 922 | 938 | 29.93 | 0.678 |
Mental disorder: includes depression and psychoses; Cancer: includes lymphoma, metastatic Cancer, and solid tumor without metastasis; Diabetes includes insulin dependent and insulin independent diabetes;
Table 9: NY-SID Prevalence of Pre-existing Comorbidities.
| Comorbidity | General Anesthesia | Regional Anesthesia | P-value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Alcohol | 163 | 1.17 | 48 | 1.53 | 0.108 |
| Deficiency Anemia | 810 | 5.82 | 135 | 4.29 | 0.001 |
| Rheumatoid Arthritis/ Collagen Vascular Disease | 313 | 2.25 | 48 | 1.53 | 0.011 |
| Chronic Blood Loss Anemia | 34 | 0.24 | 11 | 0.35 | 0.333 |
| Congestive Heart Failure | 749 | 5.38 | 163 | 518 | 0.693 |
| Chronic Pulmonary Disease | 2987 | 21.47 | 660 | 20.99 | 0.563 |
| Coagulopathy | 139 | 1.00 | 25 | 0.79 | 0.313 |
| Drug Abuse | 49 | 0.35 | 6 | 0.19 | 0.167 |
| Hypertension | 11444 | 82.25 | 2573 | 81.81 | 0.553 |
| Liver Disease | 89 | 0.64 | 29 | 0.92 | 0.095 |
| Fluid / Electrolyte Disorders | 711 | 5.11 | 87 | 2.77 | < 0.001 |
| Obesity | 771 | 5.54 | 196 | 6.23 | 0.135 |
| Paralysis | 27 | 0.19 | 4 | 0.13 | 0.642 |
| Peripheral Vascular Disorders | 2405 | 17.29 | 575 | 18.28 | 0.185 |
| Pulmonary Circulation Disorders | 32 | 1.02 | 34 | 1.08 | 0.902 |
| Renal Failure | 219 | 6.99 | 238 | 7.59 | 0.382 |
| Valvular Disease | 231 | 7.37 | 246 | 7.85 | 0.505 |
| Weight Loss | 4 | 0.13 | 8 | 0.26 | 0.387 |
| Mental Disorder | 199 | 6.35 | 213 | 6.80 | 0.508 |
| Cancer | 43 | 1.37 | 49 | 1.56 | 0.600 |
| Diabetes | 922 | 29.42 | 938 | 29.93 | 0.678 |
Mental disorder: includes depression and psychoses; Cancer: includes lymphoma, metastatic Cancer, and solid tumor without metastasis; Diabetes includes insulin dependent and insulin independent diabetes;
The overall inpatient mortalities were 0.26% and 0.10% in the GA and RA groups (P=0.226), which were lower than the 30 days mortality rate in NSQIP data as expected (Table 10). RA group was associated with lower incidences of aspiration (0.19% vs 0.61%, P=0.014), pulmonary resuscitation procedure including reintubation and ventilator support after surgery (0.54% vs 1.02%, P=0.044). There was no difference of myocardial infarction (0.61% vs 0.83%, P=0.370) in the NY-SID data.
Table 10: NY-SID Incidences of Post-admission Complications before and after Propensity Score Matching
| Before Propensity Score Matching | After Propensity Score Matching | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GA(N=13913) | RA(N=3145) | GA(N=3134) | RA(N=3134) | |||||||
| Variable Label | N | % | N | % | P-value | N | % | N | % | P-value |
| Mortality | 43 | 0.31 | 3 | 0.10 | 0.035 | 8 | 0.26 | 3 | 0.10 | 0.226 |
| Stroke | 33 | 0.24 | 5 | 0.16 | 0.531 | 4 | 0.13 | 5 | 0.16 | 1 |
| Paralysis | 62 | 0.45 | 7 | 0.22 | 0.086 | 12 | 0.38 | 7 | 0.22 | 0.359 |
| Other Neurologic Disorder | 66 | 0.47 | 10 | 0.32 | 0.299 | 13 | 0.41 | 10 | 0.32 | 0.677 |
| Aspiration | 73 | 0.52 | 6 | 0.19 | 0.012 | 19 | 0.61 | 6 | 0.19 | 0.014 |
| Respiratory Failure Pulmonary Resuscitation | 302 | 2.17 | 40 | 1.27 | 0.001 | 49 | 1.56 | 40 | 1.28 | 0.393 |
| Procedure | 178 | 1.28 | 17 | 0.54 | < 0.001 | 32 | 1.02 | 17 | 0.54 | 0.044 |
| Cardiac Arrest Cardiac Resuscitation | 284 | 2.04 | 57 | 1.81 | 0.438 | 54 | 1.72 | 57 | 1.82 | 0.848 |
| Procedure | 31 | 0.22 | 4 | 0.13 | 0.384 | 2 | 0.06 | 4 | 0.13 | 0.667 |
GA: General anesthesia; RA: Regional anesthesia;
Discussion
Our study using prospectively collected NSQIP data of 38, 102 CEA patients and NY-SID data of 17, 058 CEA patients suggests that regional anesthesia was associated with better outcome indicated by some of the complication indexes.
Why the current study is needed?
There were many studies comparing general anesthesia and regional anesthesia in CEA. However, most studies were limited by sample size to be conclusive. Meta-analysis could potentially draw conclusions to this debate. However, the heterogeneity of these studies could not adequately power the meta-analysis to draw convincing conclusion. A meta-analysis with 48 studies in 2007, including 14 prospective and 34 retrospective studies [16] concluded that, lower incidences of death, stroke, and myocardial infarction in patients receiving RA despite of the limited study power due to the low number of prospective studies [16]. However, the multicenter randomized prospective control trial, GALA trial with total of 3526 patients, found no differences in mortality, stroke, myocardial infarction, or length of hospital stay [9]. A recent meta-analysis of 14 trials and 4596 operations noticed there were lower incidences of stroke and mortality in RA group compared to GA group, while the differences were not statistically significant [17]. We took advantage of the two available large databases to address this debate on anesthesia type and surgical outcome in CEA.
Our results support these previous reports that there were no differences in mortality and stroke risks between GA and RA groups. NSQIP data showed significant lower incidence of myocardial infarction. However, NY-SID data did not support the hypothesis of lower incidence of myocardial infarction. This is likely due to the fact that NY-SID database only included ICD-9-CM code information to the point of hospital discharge, and thus potentially missed later onset complications, such as myocardial infarction, which were routinely monitored in the NSQIP data collection process. Nonetheless, our analysis on two large databases with 38,102 and 17,058 CEA operations provided valuable information on incidence of myocardial infarction at a much larger scale.
Pulmonary complications have not been well studied. Our analysis indicated differences in risks of unplanned intubation after surgery between GA and RA in the NSQIP data. Our analysis also verified the lower incidence of unplanned intubation/ prolonged ventilator support in the RA group in the NY-SID data. Furthermore, NY-SID data indicated RA group is associated with lower incidence of aspiration risk. Unfortunately, the incidence of aspiration could not be studied in the NSQIP data due to lack of such information. To our best knowledge, this is the first study with special focus on pulmonary complications. While the overall mortality and stroke risks were similar between GA and RA patients, these secondary postoperative complications could have significant implications on the requirement of perioperative resources, the length of hospitalization, and the quality of life of patients. The difference might cast significant socioeconomic influence on the patient and health care system.
Limitations of the study
The authors acknowledge that the conclusion of this study is limited due to the retrospective nature of this study design. The coding system of anesthesia type in the NSQIP and NY-SID databases is also a significant limitation. In case of concurrent use of general anesthesia with any other type(s) of anesthesia, patients were coded as receiving general anesthesia. Therefore, patients who were initially planned for RA and converted to GA might represent intraoperative complications that would show up as postoperative complications and thus be miss-assigned with anesthesia type. The NSQIP database also removed hospital and surgeon identification information in order to comply with participation agreement between NSQIP and participating sites. However, this information might be of interest to adjust relative risks. Similarly, there are limitations in the NY-SID database. NY-SID contains encounter-level information, and the integrity of the data relies on the accuracy of ICD-9-CM coding. Although many studies have validated the reliability of ICD-9-CM coding algorithm in outcome studies, the retrospective nature limited the study power.
Conclusion
Our study showed that regional anesthesia was associated with lower incidences of unexpected intubation and pulmonary resuscitation procedure after CEA compared to general anesthesia. The study of two large independent databases, NSQIP database and NY-SID database, provided more evidence on the potential beneficial effect of regional anesthesia on pulmonary complications among CEA patients. However, the choice of type of anesthesia for CEA should also be based on surgeon's recommendation and patient's preference considering the limited benefit with regional anesthesia.
Acknowledgement
The authors would like to thank Dr. Stanley Muravchick for reading the manuscript and valuable feedback.
Running title
Regional Anesthesia for CEA with Less Complication
Disclosures
The study was supported by departmental fund to JL at the University of Pennsylvania. MDN receives fund from National Institute on Aging (K08AG043548-01). The authors declare no conflict of interest.
NSQIP disclosure
"The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors."
References
- Bowyer MW, Zierold D, Loftus JP, Egan JC, Inglis KJ, Halow KD. Carotid endarterectomy: a comparison of regional versus general anesthesia in 500 operations. Annals of vascular surgery 2000;14:145-51.
- Mofidi R, Nimmo AF, Moores C, Murie JA, Chalmers RT. Regional versus general anaesthesia for carotid endarterectomy: impact of change in practice. The surgeon : journal of the Royal Colleges of Surgeons of Edinburgh and Ireland 2006;4:158-62.
- Sternbach Y, Illig KA, Zhang R, Shortell CK, Rhodes JM, Davies MG, Lyden SP, Green RM. Hemodynamic benefits of regional anesthesia for carotid endarterectomy. Journal of vascular surgery 2002;35:333-9.
- Watts K, Lin PH, Bush RL, Awad S, McCoy SA, Felkai D, Zhou W, Nguyen L, Guerrero MA, Shenaq SA, Lumsden AB. The impact of anesthetic modality on the outcome of carotid endarterectomy. American journal of surgery 2004;188:741-7.
- Lutz HJ, Michael R, Gahl B, Savolainen H. Local versus general anaesthesia for carotid endarterectomy improving the gold standard ? European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery 2008;36:145-9; disussion 50-1.
- Ferrero E, Ferri M, Viazzo A, Ferrero M, Gaggiano A, Berardi G, Pecchio A, Piazza S, Cumbo P, Nessi F. Carotid endarterectomy: comparison between general and local anesthesia. Revision of our experience with 428 consecutive cases. Annals of vascular surgery 2010;24:1034-7.
- Kasprzak PM, Altmeppen J, Angerer M, Mann S, Mackh J, Topel I. General versus locoregional anesthesia in carotid surgery: a prospective randomised trial. VASA Zeitschrift fur Gefasskrankheiten 2006;35:232-8.
- Gurer O, Yapici F, Enc Y, Cinar B, Ketenci B, Ozler A. Local versus general anesthesia for carotid endarterectomy: report of 329 cases. Vascular and endovascular surgery 2003;37:171-7.
- Lewis SC, Warlow CP, Bodenham AR, Colam B, Rothwell PM, Torgerson D, Dellagrammaticas D, Horrocks M, Liapis C, Banning AP, Gough M, Gough MJ. General anaesthesia versus local anaesthesia for carotid surgery (GALA): a multicentre, randomised controlled trial. Lancet 2008;372:2132-42.
- Leichtle SW, Mouawad NJ, Welch K, Lampman R, Whitehouse WM, Jr., Heidenreich M. Outcomes of carotid endarterectomy under general and regional anesthesia from the American College of Surgeons' National Surgical Quality Improvement Program. Journal of vascular surgery 2012;56:81-8 e3.
- Schechter MA, Shortell CK, Scarborough JE. Regional versus general anesthesia for carotid endarterectomy: the American College of Surgeons National Surgical Quality Improvement Program perspective. Surgery 2012;152:309-14.
- Romano PS, Chan BK, Schembri ME, Rainwater JA. Can administrative data be used to compare postoperative complication rates across hospitals? Medical care 2002;40:856-67.
- Silber JH, Romano PS, Rosen AK, Wang Y, Even-Shoshan O, Volpp KG. Failure-to-rescue: comparing definitions to measure quality of care. Medical care 2007;45:918-25.
- Matsen SL, Chang DC, Perler BA, Roseborough GS, Williams GM. Trends in the in hospital stroke rate following carotid endarterectomy in California and Maryland. Journal of vascular surgery 2006;44:488-95.
- Neuman MD, Silber JH, Elkassabany NM, Ludwig JM, Fleisher LA. Comparative effectiveness of regional versus general anesthesia for hip fracture surgery in adults. Anesthesiology 2012;117:72-92.
- Guay J. Regional or general anesthesia for carotid endarterectomy? Evidence from published prospective and retrospective studies. Journal of cardiothoracic and vascular anesthesia 2007;21:127-32.
- Vaniyapong T, Chongruksut W, Rerkasem K. Local versus general anaesthesia for carotid endarterectomy. Cochrane Database Syst Rev 2013;12:CD000126.