Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/032
ORIGINAL RESEARCH OPEN ACCESS
A randomized, placebo-controlled, concealed allocation comparison of respiratory depression during bronchoscopy with dexmedetomidine-ketamine as an adjunct to fentanyl-midazolam sedation
Joshua H. Atkins1 , MD, PhD, Andrew R. Haas2, MD, Daniel H. Sterman3, MD, Anil Vachani MD2, MS, Jeff E Mandel1, MD, MS
1Department of Anesthesiology and Critical Care, Perelman School of Medicine at the University of Pennsylvania
2Pulmonary, Allergy and Critical Care Division, Perelman School of Medicine at the University of Pennsylvania
3Department of Medicine, New York University Langone Medical Center
Joshua Atkins, MD, PhD, Assistant Professor; Department of Anesthesiology and Critical Care, Perelman School of Medicine at the University of Pennsylvania, Dulles 680, 3400 Spruce Street, Philadelphia, PA 19104 USA, Phone: 1 215 203 4781, FAX: 1 215 349 8133, Email: Joshua.Atkins@uphs. upenn.edu
Editor: Yuan-Xiang Tao, MD, PhD Department of Anesthesiology Rutgers New Jersey Medical School, Rutgers, The State University of New Jersey 185 S. Orange Ave., MSB, F-548, Newark, NJ 07103. Tel: +1-973-972-9812; Fax: +1-973-972-1644 Email: yuanxiang.tao@njms.rutgers.edu
Received: August 13, 2015 | Accepted: September 20, 2015 | Published: December 08, 2015
Citation: Atkins JH, Haas AR, Sterman DH, Vachani A, Mandel JE. A randomized, placebo-controlled, concealed allocation comparison of respiratory depression during bronchoscopy with dexmedetomidine-ketamine as an adjunct to fentanyl-midazolam sedation.Transl Perioper & Pain Med 2016; 1(1): 24-31.
Abstract
Introduction
During advanced bronchoscopic procedures, adequate sedation is required to obtain patient cooperation. Sedation with conventional agents such as midazolam and fentanyl carries a risk of respiratory depression. Dexmedetomidine and ketamine are associated with minimal respiratory depression. We hypothesized that the combination of these agents would reduce the requirement for fentanyl and midazolam and decrease respiratory depression with similar patient and proceduralist satisfaction.
Methods
50 patients undergoing flexible bronchoscopy and curvilinear probe endobronchial ultrasound were randomly assigned to receive dexmedetomidine/ketamine or placebo. Supplemental fentanyl and midazolam could be requested by the proceduralist. Ventilation was assessed by respiratory inductance plethysmography (RIP), arterial saturation by pulse oximetry, and satisfaction by the Likert scale. Patients, proceduralists, and anesthesiologists were blinded to allocation. Midazolam and fentanyl plasma concentrations were estimated from dosing history using validated pharmacokinetic models, relative minute ventilation derived from RIP, and desaturation by cumulative time below 90%.
Results
Groups were similar. Patients in the experimental group achieved lower mean fentanyl and midazolam levels. Satisfaction scores and time below oxygen saturation of 90% were not different between groups. The relative decrease below baseline minute volume was greater in the control group 0.736 (0.566 - 0.848) compared to experimental 0.764 (0.592 - 0.891), P < 0.0001.
Conclusions
The addition of dexmedetomidine and ketamine to midazolam/fentanyl sedation reduced the requirement for these agents, did not alter satisfaction or oxygen desaturation, but did reduce the magnitude of decrease in minute ventilation. These effects may be greater in an open label study.
Keywords
Procedural sedation, respiratory depression, bronchoscopy
Bronchoscopy is a stimulating procedure that involves transit of the fiberscope through the oropharynx and across the vocal cords and contact with the tracheobronchial mucosa. The use of local anesthetic with sedation or general anesthesia is considered the standard of care [1]. Sedation with midazolam has been associated with increased patient reported tolerance of bronchoscopy [2], but led to an increased rate of oxygen desaturation compared to placebo. Decreases in minute volume may be far more common, as desaturation is masked by the use of supplemental oxygen [3].
The practice of bronchoscopy has undergone considerable evolution in recent years due to the influx of new technology. Endobronchial ultrasound (EBUS), for example, has made minimally invasive biopsy of mediastinal nodes feasible. With the introduction of EBUS and other novel techniques, however, the time required for bronchoscopy has been extended, and the ability of patients to tolerate these procedures under conventional procedural sedation is increasingly being tested. While the most complex cases are being performed in operating rooms with general anesthesia, Casal and colleagues failed to demonstrate any improvement in diagnostic yield between moderate sedation with midazolam and fentanyl and deep sedation/general anesthesia with propofol and remifentanil [4]. It is likely that many procedures will continue to be performed under conscious sedation, and these techniques should evolve to keep pace with the evolution in procedures.
How can we define quality in sedation? The purpose of sedation is to permit patient compliance with the intended procedure with a minimal derangement in physiologic function. When using agents that depress respiratory drive, a balance must be struck between adequate conditions for safe completion of the procedure and avoidance of over-sedation. This balance may be shifted by periodic stimulation of the patient to breathe and maneuvers to ameliorate obstruction. Sedative medications that do not depress respiration permit the safe accomplishment of procedures that would otherwise require general anesthesia.
Dexmedetomidine (DEX) is a selective α-2 receptor agonist approved in the United States for sedation during procedures and for patients in the intensive care unit. DEX has a limited side-effect profile, with modest hypotension and bradycardia being the most common side effects seen in case series [5]. As a single agent DEX causes minimal respiratory depression, however airway reflex suppression and amnesia is unreliable [6]. These limitations may be addressed by the combination of DEX with ketamine [7], as this combination has been associated with dissociative amnesia and analgesia without changes in respiratory rate [8]. DEX is antisialagogic, countering the sialagogic properties of ketamine. While the DEX-ketamine combination has been found to reduce the need for supplemental midazolam to obtain immobility during sedation for pediatric MRI [9], the effect of this combination on the requirement for additional sedative hypnotics such as midazolam and fentanyl and the impact on ventilation has not been adequately studied. We hypothesized that the combination of these agents would reduce the requirement for fentanyl and midazolam, with a lower incidence of respiratory depression, at equivalent levels of proceduralist and patient satisfaction.
Materials and Methods
With approval of the Institutional Review Board of the University of Pennsylvania School of Medicine (FWA00004028) and written informed consent, 50 patients scheduled for endobronchial ultrasound procedures under conscious sedation were enrolled in a prospective, concealed allocation study. The study was registered with Clinicaltrials.gov (NCT01158820), and all elements of the CONSORT version 4 checklist were addressed.
Inclusion criteria were patients over the age of 18 scheduled for flexible bronchoscopy with curvilinear (or convex-probe) endobronchial ultrasound guided biopsy of mediastinal or paramediastinal nodes or masses under conscious sedation.
Exclusion criteria were:
History of inability to complete bronchoscopy attributable to inadequate sedation.
Requiring more than 2 LPM supplemental oxygen to maintain pre-procedure SaO2 > 90% at baseline.
History of allergy to study medications
Pregnancy1
A history of psychosis
Any condition deemed likely by the pulmonologist or anesthesiologist to pose a significant risk due to elevation of blood pressure, including cerebral/aortic aneurysm, and or ischemic cardiovascular disease.
History of bradydysrhythmia deemed significant by the anesthesiologist or pulmonologist
History of significant renal or hepatic impairment
Inability to provide informed consent
All patients were screened and recruited by an anesthesiologist investigator (JHA or JEM) who was present for the entire procedure without other clinical assignments. Consent was obtained immediately prior to the procedure. Two patients were excluded; one for renal failure, another for insufficient fluency in English to permit informed consent. An additional 47 patients declined to participate.
The primary outcome variable was change in minute ventilation, as assessed by calibrated respiratory inductance plethysmography. Secondary outcome variables included estimated plasma concentrations for fentanyl and midazolam, pulmonologist and patient satisfaction. Primary safety endpoints included:
Desaturation < 85% persisting for 60s
Hypotension (Mean BP < 60) unresponsive to pressors (ephedrine, phenylepherine)
Bradycardia (HR < 45) unresponsive to atropine or epinephrine.
Persistent coughing unresponsive to supplemental lidocaine
Inadequate sedation precluding safe conduct of the procedure, as assessed by the pulmonologist.
Subjects were randomized to one of two arms of the protocol with concealed allocation. Patients, proceduralists and anesthesiologists were unaware of group assignment. This was accomplished using 5 syringes, as listed in Table 1. In both arms, nebulized lidocaine (maximum dose, 200 mg) was administered prior to initiation of sedation.
Table 1: Syringe contents
| Control | Experimental | |
|---|---|---|
| Syringe A | Saline 50 ml | Dexmedetomidine 200 µg |
| Syringe B | Midazolam 2 mg + Fentanyl 50 µg | Ketamine 30 mg |
| Syringe C | Saline 20 ml | Ketamine 200 mg |
| Syringe D | Midazolam 10 mg + Fentanyl 250 µg + saline 5 ml | |
| Syringe E (2) | Diphenhydramine 25 mg |
Contents of the 5 syringes utilized in the concealed allocation.
Syringe A was administered at a rate of 0.25 ml/kg over 10 minutes following completion of nebulization (1 µg/kg dexmedetomidine in the experimental arm). Syringe B was administered subsequent to completion of the loading dose from syringe A, with the infusion rate of syringe A lowered to 0.175 ml/kg/hr (0.5 µg/ kg/hr dexmedetomidine in the experimental arm), and an infusion of 0.8 µl/kg/min of Syringe C initiated (8 µg/kg/min ketamine in the experimental arm). Syringe D was loaded in a Graseby 3300 PCA pump. The serial output of the PCA pump was connected directly to the data acquisition system, permitting accurate timing of demand boluses. The anesthesiologist investigator pressed the button at the request of the pulmonologist. Syringe E was administered after the 6th and 16th bolus of Syringe D. Syringes were prepared by the Investigational Drug Service of the Hospital of the University of Pennsylvania and dispensed after patient consent was obtained. Plasma concentrations of fentanyl and midazolam were estimated using the validated pharmacokinetic models of Shafer and Somma [10,11].
All patients were fitted with respiratory inductance plethysmography (RIP) bands (Respitrace, Ambulatory Monitoring Inc, Armonk, NY). A pneumotachograph (Hans Rudolph, St. Louis, MO) was used to calibrate the RIP. Prior to each study, the pneumotachograph was calibrated with a 3 liter air syringe. RIP calibration was derived from a period immediately prior to or immediately following the procedure using methods described in detail by Mandel et al. [12] In brief, RIP and pneumotachograph signals were analyzed using the Hilbert-Huang transform to yield instantaneous magnitude estimates, and multiple linear regression was performed to obtain coefficients to predict minute volume from RIP signals. Baseline minute ventilation was obtained from the minute prior to initiation of sedation. All minute ventilation values were scaled by the baseline value; 1.0 indicates no change from baseline. We excluded values above 1.0 as patients exhibiting respiratory depression are typically coached to take a deep breath following the observation of hypoventilation, and patients experiencing obstruction will exhibit a compensatory hyperpnea following relief of obstruction. Estimates consistent with respiratory depression (relative minute volume < 1.0) for the period from administration of Syringe B until one hour later (or conclusion of the procedure) were analyzed.
Data was sampled at 240 Hz using a 12 bit analog-to-digital converter, and signals acquired and analyzed using custom software implemented in LabVIEW 2011 (National Instruments, Austin, TX). Pulse oximetry was performed using a Masimo Radical pulse oximeter; data was acquired at 1 second intervals by RS-232 serial output. Pulmonologist and patient satisfaction scores were assessed by 11 point Likert scale.
Sedation scores were not assessed directly, but pulmonologists were instructed to request a button press whenever they felt the patient was inadequately sedated. Pulmonologists were also free to request conversion to general anesthesia if they felt the patient was not tolerating the procedure under the sedation regimen, and were not required to justify their decision.
Statistical analysis was performed using the Statistics Toolbox in MATLAB 2015a (Mathworks, Natick, MA). Normally distributed data was compared by 2 sample unpaired T test and reported as mean ± SD; categorical data by Fisher's exact test, and non-normally distributed data by Mann-Whitney U test and reported as median and interquartile range. P< 0.01 was considered significant.
Sample size calculation was based on the results of Chhajed et al. that assessed the ability to detect respiratory depression during sedation with midazolam using transcutaneous CO measurements, demonstrating a mean baseline 36 ± 8 mmHg that increased to 46 ± 9 mmHg during sedation [13]. We assumed a linear relationship between changes in minute volume and CO . Thus, a study with 48 patients would have a power of 80.7% to observe this large a change in minute volume if each patient represented a single observation. Our methodology utilized instantaneous estimates of minute ventilation under changing conditions of stimulation and midazolam/fentanyl levels, thus each patient could be represented by multiple observations, increasing the statistical power above that which would be expected for Chhajed's design.
Results
50 patients were enrolled, however, all data was lost from one patient in the control arm due to a computer malfunction. In an additional 5 patients (one in control and 4 in experimental), RIP signal loss [3], artifacts [1], or inadequate time prior to conversion to general anesthesia [1] precluded obtaining useful estimates of minute volume.
The groups were comparable in age, height, weight, sex, the number of patients with ASA 3 physical status, and baseline oxygen saturation. Anthropomorphic data is summarized in Table 2.
Table 2: Patient characteristics
| Control | Experimental | P | |
|---|---|---|---|
| Age (yrs) | 59.4 ± 13.2 | 61.2 ± 14.9 | 0.667 |
| Weight (lbs) | 174.4 ± 44.6 | 182.6 ± 42.1 | 0.507 |
| Height (in) | 67.2 ± 4.5 | 68.0 ± 3.0 | 0.457 |
| Sex (F/M) | 13/12 | 6/19 | 0.079 |
| ASA (3/1-2) | 6/19 | 7/18 | 1.000 |
| Baseline Saturation (%) | 97.5 ± 2.3 | 97.4 ± 2.5 | 0.815 |
| Procedure duration (min) | 58.6 ± 28.9 | 58.3 ± 20.7 | 0.968 |
Age, weight, height, saturation, and procedure duration are reported as mean ± std. dev., with p determined by 2 sample unpaired t test. p values for sex and ASA status were determined by Fisher's exact test.
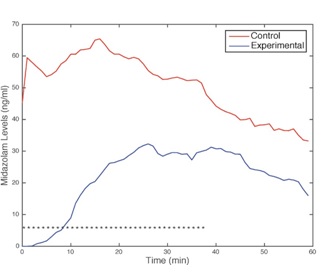
Patients in the control group were administered larger amounts of fentanyl (112.5 vs. 68.75 µg) and midazolam (4.50 vs. 2.75 mg), yielding average plasma concentration of 1.0 vs. 0.7 ng/ml fentanyl and 52.7 vs. 38.9 ng/ml, both significant at p = 0.01. No significant correlation was observed between midazolam/fentanyl levels and age, weight, height, or baseline saturation. The trajectory of midazolam plasma levels for the two groups is depicted in Figure 1. Asterisks indicate a difference at p < 0.05 between groups (calculated at one minute intervals). Experimental group patients received significantly less midazolam (and fentanyl) for the first 37 minutes of the procedures; beyond this time, differences were not statistically significant, primarily due to smaller numbers of patients present in the comparisons. The number of patients receiving diphenhydramine 25 mg [9] and 50 mg [2] were identical between groups.
Figure 1: Mean midazolam plasma estimates for the two groups. * indicates p < 0.05 difference by Mann-Whitney U test for calculated at one minute intervals.
Pulmonologist and patient satisfaction ratings were similar; 7 vs. 8 (p = 0.4) and 9 vs. 10 (p = 0.64). Regarding primary safety endpoints, no patients experienced desaturation < 85% exceeding 60s. No patients required intubation or bag-mask ventilation to resolve hypoxia. The average time below a saturation of 90% was not significantly different; 39 vs. 40 seconds (p = 0.818). Patients in the experimental group were more likely to be treated for hypotension (p = 0.049), but all patients responded to ephedrine or phenylephrine. No patients required treatment for bradycardia. Patients in the experimental group were less likely to require conversion to general anesthesia (consisting of LMA and total intravenous anesthesia) (p = 0.098). Proceduralists were not required to state a reason for conversion to general anesthesia.
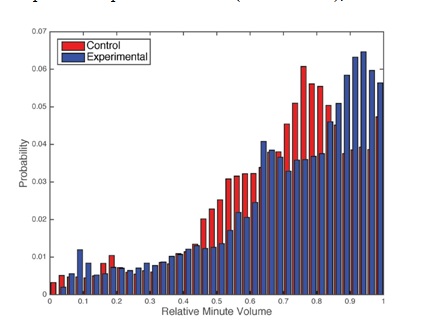
Relative minute volume averaged over the entire procedure was not significantly different between groups; 0.86 (0.75 – 1.12) vs. 0.85 (0.78 – 1.09), p = 1.0. The distribution of samples with relative minute volume below 1.0 is depicted in Figure 2. Patients in the control group exhibited a greater frequency of respiratory depression; 0.736 (0.566 - 0.848) compared to experimental 0.764 (0.592 - 0.891), P < 0.0001.
Figure 2: Histogram of relative minute volumes below 1.0. X axis is relative minute volume, Y axis is the proportion of observations in the bin.
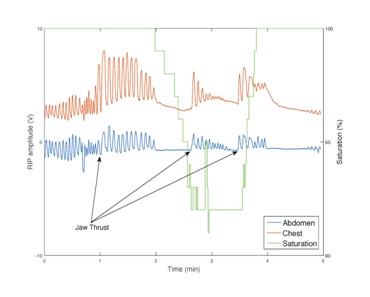
A 5 minute sample of data from a 30 year old, 78 kg patient in the control group is presented in Figure 3 to clarify this result. Prior to the beginning of the epoch, the patient had received 3.5 mg of midazolam and 87.5 µg of fentanyl, with plasma level estimates 56.2 ng/ml and 1.06 ng/ml, slightly above the median values seen in the control group. During the initial 10 breaths, the patient exhibits normal ventilation, but subsequently is felt to be obstructed. As indicated by arrows, the proceduralist performs jaw thrust on three occasions, with a transient increase in chest and abdominal excursion above baseline. These increases are short-lived, and periods of apnea follow each of these interventions. Despite this, the nadir of saturation is 92%. The mean ventilation across the epoch is normal, and yet a significant derangement in ventilation has occurred.
Figure 3: A 5 minute epoch from a patient in the control group illustrating obstructed ventilation. The first 10 breaths are close to basal minute ventilation, but jaw thrust is applied thrice, with increases in chest and abdominal excursion above basal levels. While saturation transiently decreases, the nadir is approximately 92%.
Discussion
Respiratory depression during advanced bronchoscopic procedures is problematic. Significant desaturation necessitates scope removal and prolongs the procedure. Subsequent resuscitation may be more difficult in the patient with underlying lung pathology such as chronic obstructive pulmonary disease or obstructive sleep apnea. Midazolam and fentanyl are commonly employed for sedation during bronchoscopy but the combination synergistically depresses respiration [14]. It is difficult to predict the respiratory response of an individual patient to a subsequent bolus of these drugs and monitors of respiration under these conditions have limited reliability. The American Society of Anesthesiologists analysis of data from closed medical malpractice cases reflects a significant risk of morbidity from hypoventilation during sedation [15]. Patients on chronic opioid or benzodiazepine therapy may be more difficult to sedate while those with obstructive sleep apnea may be at higher risk of sleep disordered breathing in the perioperative period. For all of these reasons, alternative sedation regimens with the potential to decrease the dose of respiratory depressant medications are desirable.
Our study demonstrates that the combination of dexmedetomidine and ketamine significantly decreased the dose of both midazolam and fentanyl administered during advanced bronchoscopic procedures. This decrease was associated with a diminution in the severity of transient episodes of hypoventilation, as quantified by continuous monitoring of minute ventilation. While the average minute ventilation was not different between groups, exclusion of minute volumes above baseline demonstrated a greater degree of respiratory depression in the control group. This reflected the tendency in control patients to transiently decrease their minute ventilation, leading the proceduralist to stimulate the patient to breathe or perform chin lift or jaw thrust. The ensuing period of hyperpnea may mask the preceding period of hypopnea when averaging over longer periods of time, confounding detection by pulse oximetry or transcutaneous CO, as was illustrated in Figure 3. Without an occlusive seal such as an endotracheal tube, minute ventilation is not typically measured during bronchoscopy. Using RIP and the Hilbert-Huang transform permitted nonintrusive assessment of minute ventilation with high temporal resolution, and is the novel aspect of this effort. Although these transient periods of hypoventilation did not produce clinically significant complications in our small sample, it is possible that in a larger sample, or a cohort with more severe lung disease, complications could be observed. While it is difficult to assess the benefit of a lower requirement to coach patients to breathe or relieve obstruction during advanced bronchoscopic procedures, this may be important in some patients and convenient in many. Propofol incurs the requirement for a practitioner skilled in airway management who is not involved in performance of the procedure, which may provide motivation for employing moderate sedation, DEX-ketamine may further improve patient safety when this resource is not available.
Overall the study supports the use of dexmedetomidine-ketamine as an adjunct to benzodiazepine-opioid conscious sedation regimens for bronchoscopy by demonstrating equivalence of patient and proceduralist satisfaction, general anesthesia conversion rate, and safety end points of desaturation. While in most patients, the difference in respiratory depression was small between the two groups, this difference might be clinically relevant in a subset of patients, particularly those with underlying COPD and OSA. The methods utilized in this study should not be viewed as a template for routine clinical care, or even for an open label study. Just as the midazolam and fentanyl were titrated to effect in this study, DEX and ketamine would be titrated to effect in normal clinical practice. While the use of infusion pumps may seem needlessly complex to clinicians accustomed to hand delivered boluses, there may also be advantages to a sedation approach that relies on continuous steady state infusions or fixed ratio bolus dosing from a pump. The data support the further investigation of this methodology and the dexmedetomidine-ketamine regimen for proceduralist directed sedation.
The study has a number of limitations. All sedation was provided under the supervision of an anesthesiologist. Pulmonologists may have altered their sedation practices, either being more aggressive in some cases, or abandoning sedation for general anesthesia in others. The study population was too small to permit subgroup analysis. It is possible that larger differences in respiratory parameters could be detected if higher risk groups are specifically studied. Additionally, due to the concealed allocation, provisions were made to assure that patients in the control arm were able to receive adequate sedation through demand administration of fentanyl and midazolam, rather than adjustments in study drug. Adjustments in dexmedetomidine and/or ketamine may have yielded acceptable sedation with less respiratory depression, but it was deemed unethical to treat inadequate sedation with placebo. It is quite possible that an open label protocol would find larger differences in respiratory depression.
No assessment of OAS/S scores was made; it was assumed that proceduralists (who had extensive experience performing these procedures with conscious sedation) would regulate sedation based on their assessment of patient requirements. While addition of dexmedetomidine to midazolam sedation for awake fiberoptic intubation did not significantly alter OAS/S scores, significant differences were seen in assessments of patient calmness and comfort [16]. We did not attempt to assess the reasons the proceduralists requested additional sedation. Again, these limitations would be less apparent in an open label study or routine clinical practice.
In conclusion, the use of DEX-ketamine in conscious sedation for advanced bronchoscopic sedation may reduce the requirement for other agents associated with ventilatory depression. Clinicians comfortable with conscious sedation employing fentanyl and midazolam will find DEX-ketamine to provide conditions that are at worst equivalent, and in many cases superior to their normal routine.
Conflict Interests Disclosure
The authors have no conflicting interests to disclose.
Disclosure of Funding
None.
References
- Wahidi MM, Jain P, Jantz M, et al. American College of Chest Physicians consensus statement on the use of topical anesthesia, analgesia, and sedation during flexible bronchoscopy in adult patients. Chest. 2011;140(5):1342-50.
- Contoli M, Gnesini G, Artioli D, et al. Midazolam in flexible bronchoscopy premedication: effects on patient-related and procedure-related outcomes. J Bronchology Interv Pulmonol. 2013;20(3):232-40.
- Fu ES, Downs JB, Schweiger JW, et al. Supplemental oxygen impairs detection of hypoventilation by pulse oximetry. Chest. 2004;126(5):1552-8.
- Casal RF, Lazarus DR, Kuhl K, et al. Randomized Trial of Endobronchial Ultrasound-guided Transbronchial Needle Aspiration under General Anesthesia versus Moderate Sedation. Am J Respir Crit Care Med. 2015;191(7):796-803.
- Grewal A. Dexmedetomidine: New avenues. J Anaesthesiol Clin Pharmacol. 2011;27(3):297-302.
- Hsu Y-W, Cortinez LI, Robertson KM, et al. Dexmedetomidine pharmacodynamics: part I: crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004;101(5):1066-76.
- Zanaty OM, and El Metainy SA. A Comparative Evaluation of Nebulized Dexmedetomidine, Nebulized Ketamine, and Their Combination as Premedication for Outpatient Pediatric Dental Surgery. Anesth Analg. 2015; 121(1):167-71 .
- Mester R, Easley RB, Brady KM, et al. Monitored anesthesia care with a combination of ketamine and dexmedetomidine during cardiac catheterization. Am J Ther.2008;15(1):24-30.
- Tammam TF. Comparison of the efficacy of dexmedetomidine, ketamine, and a mixture of both for pediatric MRI sedation. Egyptian Journal of Anaesthesia. 2013;29(3):241-246.
- Shafer SL, Varvel JR, Aziz N, et al. Pharmacokinetics of fentanyl administered by computer-controlled infusion pump. Anesthesiology. 1990;73(6):1091-102.
- Somma J, Donner A, Zomorodi K, et al. Population pharmacodynamics of midazolam administered by target controlled infusion in SICU patients after CABG surgery. Anesthesiology. 1998;89(6):1430-43.
- Mandel JE, and Atkins JH. Hilbert Huang transform yields improved minute volume estimates from respiratory inductance plethysmography during transitions to paradoxical breathing. Anesth Analg. in press.
- Chhajed PN, Rajasekaran R, Kaegi B, et al. Measurement of combined oximetry and cutaneous capnography during flexible bronchoscopy. Eur Respir J.2006;28(2):386-90.
- Bailey PL, Pace NL, Ashburn MA, et al. Frequent hypoxemia and apnea after sedation with midazolam and fentanyl. Anesthesiology. 1990;73(5):826-30.
- Bhananker SM, Posner KL, Cheney FW, et al. Injury and liability associated with monitored anesthesia care: a closed claims analysis. Anesthesiology. 2006;104(2):228-34.
- Bergese SD, Patrick Bender S, McSweeney TD, et al. A comparative study of dexmedetomidine with midazolam and midazolam alone for sedation during elective awake fiberoptic intubation. J Clin Anesth. 2010;22(1):35-40.