Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/088
CASE REPORT OPEN ACCESS
Leriche Syndrome in the Setting of Coronary Artery Bypass Grafting
Roy E. Henrickson, MD1, John Bozek, MD2, Kevin Tveter, MD3, Pavithra Ranganathan, MD4, Jeffrey Puette, MD1 and Matthew B. Ellison, MD4*
1Assistant Professor, Department of Anesthesiology, West Virginia University School of Medicine, 1 Medical Center Dr, Morgantown, WV, 26506, USA
2Staff Anesthesiologist, Department of Anesthesiology, Lenox Hill Hospital, 100 E 77th Street, New York, New York 10075, USA
3Assistant Professor, Heart and Vascular Institute, West Virginia University School of Medicine, 1 Medical Center Dr, Morgantown, WV, 26506, USA
4Associate Professor, Department of Anesthesiology, West Virginia University School of Medicine, 1 Medical Center Dr, Morgantown, WV, 26506, USA
Matthew B. Ellison, MD, Department of Anesthesiology, West Virginia University, PO Box 8255, 1 Medical Center Drive, Morgantown, WV, 26506, USA, Tel: 304-285-1931, Fax: 304-598-4930, E-mail: mellison@wvumedicine.org
Editor: Henry Liu, MD, MS, FASA, Professor of Anesthesiology, Vice Chairman for Research, Drexel University College of Medicine, Hahnemann University Hospital, 245 N. 15th Street, MS 310, Philadelphia, PA 19102, USA, E-mail: henryliupa@gmail.com
Received: February 15, 2019 | Accepted: February 28, 2019| Published: March 12, 2019
Citation: Henrickson RE, Bozek J, Tveter K, Ranganathan P, Puette J, et al. Leriche Syndrome in the Setting of Coronary Artery Bypass Grafting. Transl Perioper & Pain Med 2019; 6 (2):53-57.
Abstract
We present the case of a 60-year-old male with Leriche Syndrome discovered on cardiac catheterization in preparation for coronary artery bypass grafting. While the patient was in the operating room receiving sedation and preoxygenation, additional angiography was requested to determine the routes of collateralization to the lower extremities. Because no route could be established with existing angiographic images, the case was canceled before induction of anesthesia. It was later determined by further testing that collateral flow to the bilateral lower extremities was dependent on the bilateral internal mammary arteries. This patient was subject to increased morbidity due to the inability to determine collateral flow and appropriate grafting vessels prior to the scheduled operation, leading to delay in treatment.
Leriche Syndrome, or aortoiliac occlusive disease, is a known, but infrequently encountered coexisting disease process in patients presenting for coronary artery bypass grafting (CABG) procedures. Recognizing the importance of its implications specific to CABG surgery can help to avoid major postoperative morbidity and mortality through proper evaluation and surgical consideration.
Keywords
Leriche syndrome, Coronary artery bypass grafting, Aortoiliac occlusive disease, Coronary artery disease, Percutaneous coronary intervention
Case Report
We describe the case of a 60-year-old male with a past medical history of hypertension, hyperlipidemia, and extensive smoking. He was transferred to our facility with the primary complaint of angina. The initial attempt at cardiac catheterization at an outside hospital via the femoral artery was aborted when aortoiliac occlusive disease was demonstrated. After arrival to this facility, catheterization via right radial artery was successful and was consistent with multi-vessel coronary artery disease (CAD), a mildly reduced ejection fraction as well as a completely occluded infra-renal aorta. A transthoracic echocardiogram showed an ejection fraction of approximately 45%, akinesis of basal and mid interoseptal walls, and significant diastolic dysfunction. Upon presentation, the patient also experienced a history of impotence and gluteal pain with ambulation. His physical exam demonstrated absent femoral pulses as well as bilateral lower extremity atrophy and hair loss.
The patient was referred for coronary artery bypass grafting (CABG) but the plan was complicated by lack of graft conduit. Specifically, the patient was found by ultrasound to have bilateral saphenous veins and a right radial artery inadequate for grafting. In addition, there was also concern for left radial artery grafting because of left subclavian artery stenosis, which was unsuitable for stenting. Given the lack of conduit vessels, it was decided that the patient should undergo surgical grafting of the left anterior descending (LAD) artery using the left internal mammary artery (LIMA) followed by percutaneous coronary intervention (PCI) for treatment of his remaining CAD.
Preoperatively, the surgery service was queried by the anesthesiologist as to whether the routes of collateralization to the lower extremities were known. The response indicated that there had been intraabdominal collaterals demonstrated on angiography from the radial artery catheterization upon admission. The patient was then transported to the operating room, and a radial arterial line was placed during preoxygenation and prior to induction. After further consideration, the surgeon requested a delay of induction of anesthesia for further angiography review. Upon examination, a large right inferior epigastric artery was noted on the preoperative catheterization films, suggesting that collateral blood from the right internal mammary artery may supply the right lower extremity. The origin of collateral flow, however, could not be determined. Existing angiography was also unable to clearly demonstrate the collateral blood flow to the left lower extremity. At this point, the case was aborted before induction of anesthesia, and further angiography studies were ordered in consultation with a vascular surgeon. A subsequent aortogram and angiography of the internal mammary arteries demonstrated that collateral flow to the lower extremities was dependent on both the left and right internal mammary arteries (Figure 1, Figure 2 and Figure 3). Gastroepiploic artery was not considered as an alternative to the LIMA because of lack of institutional experience with the procedure.
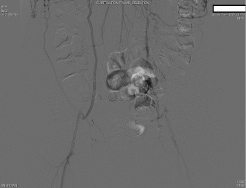
Figure 1: Angiogram demonstrating infrarenal aortic occlusion with collateralization.
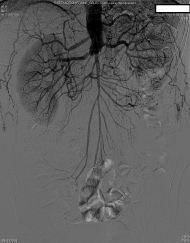
Figure 2: Angiogram demonstrating collateral flow to the lower extremities from the internal mammary arteries via the inferior epigastric arteries.
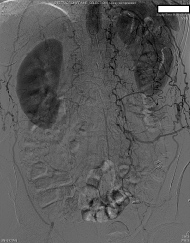
Figure 3: Angiogram demonstrating collateral flow to the lower extremities due to aortic occlusion.
With no suitable conduits, the surgery was canceled and the patient underwent staged PCI for multi-vessel CAD, including four stents to the right coronary artery (RCA), followed by two stents to the LAD and one stent to the diagonal artery for a total of seven drug-eluding stents. In addition, the vascular surgery service recommended aortobifemoral bypass shortly after recovery. The patient was discharged from the hospital in stable condition following successful placement of his multiple coronary artery stents.
Approximately four months after his initial presentation, the patient was again transferred to our facility for electrocardiogram changes suggestive of lateral ischemia. Urgent cardiac catheterization was performed utilizing the radial artery which demonstrated occluded stents in the RCA and subtotal occlusion of stents in the LAD and diagonal artery. The cardiologist noted that the ejection fraction at this time was severely reduced to 15-20%.
He was still deemed unsuitable for CABG by the cardiothoracic surgery service due to lack of conduit vessels. Following discussion with the patient regarding his options, a repeat complex PCI was planned for following morning; however, he subsequently suffered cardiac arrest that night and ultimately expired following 33 minutes of resuscitative efforts.
Discussion
In 1940, vascular surgeon René Leriche described the triad (now known as Leriche syndrome) of lower extremity claudication, impotence, and absent femoral pulses due to the occlusion of the distal abdominal aorta, possibly extending into one or both iliac arteries [1,2]. This syndrome, also known as aortoiliac occlusive disease, classically presents in males between the fourth and sixth decade of life. Although the pathophysiology is similar to that of chronic atherosclerosis with risk factors including hyperlipidemia, smoking, hypertension, and diabetes mellitus, Leriche syndrome has been described in patients whose only risk factor is an extensive smoking history [3,4].
Compared to more distal arterio-occlusive lesions (femoral, popliteal, sural, etc.), collateral blood flow is more likely to develop in the setting of aortoiliac occlusion and may originate from numerous sources, including the internal mammary artery, also known as the internal thoracic artery [1,5]. Originating from the subclavian artery, the internal mammary artery transitions into the superior epigastric artery, which anastomoses with the ipsilateral inferior epigastric artery in a mutual capillary bed. During the formation of collateral circulation due to chronic aortoiliac occlusion, flow reversal occurs in the inferior epigastric artery so that the external iliac arteries provide blood flow to the lower extremities [5]. Typically, collateral flow only develops in the setting of severe to complete aortoiliac disease because of the need to overcome the native resistance of this vascular pathway [6]. Leriche syndrome typically develops chronically, but acute forms have been described. These include acute paraplegia with presumed occlusion of the artery of Adamkiewicz, acute thrombosis of the superior mesenteric and the right renal arteries, and as part of a massive systemic thromboembolic crisis [7-9].
As early as the 1948 publication by Leriche and Morel, it was realized that neurologic symptoms of the lower extremities were potentially misleading signs of symptoms of this vascular process [2]. More recent studies have further emphasized this point, since a compromised blood supply to affected nerves may lead to abnormalities on physical exam and on nerve conduction studies [10]. For this reason, a high index of suspicion for vascular causes of lower extremity neurologic symptoms must be maintained in the proper clinical circumstances [2]. Physical exam may reveal atrophy of the lower extremities and decreased lower extremity pulses [1]. Observing patients' physical endurance in studies such as the six-meter walk test or exercise stress testing may help differentiate claudication that is vascular versus neurologic in origin [1]. The workup may also be supplemented by ankle-brachial index measurements, doppler ultrasonography to assess the hemodynamics of aortoiliac blood flow, and arteriography [1].
The classic treatment for Leriche syndrome, aortobifemoral bypass, is less commonly performed due to less invasive options including conservative management, aortoiliac endarterectomy, extra-anatomical bypass grafts, and endovascular bypass [1]. Given the common risk factors and etiology, coronary artery disease (CAD) and peripheral vascular disease (PVD) are often coexistent. As with our patient, Leriche syndrome is sometimes diagnosed during cardiac catheterization for co-existing CAD, often because of an inability to gain intra-arterial femoral access during catheterization [3]. Routine angiography of the LIMA during cardiac catheterization is controversial, possibly because of conflicting evidence in the literature. Feit, et al. utilized routine LIMA angiography during cardiac catheterization in 130 patient, revealing significant findings in 15% of the subjects [11]. A more recent study by Patel, et al. showed that significant disease of the LIMA was rare and that preoperative subclavian artery stenosis did not correlate with postoperative coronary artery ischemia [12]. The authors believe that concomitant angiography of the LIMA during cardiac catheterization should be performed in patients who meet the following criteria: Patients who have had a previous mastectomy and radiation therapy, patients who are suspected to have or have a history of lower extremity peripheral vascular disease and are being referred for CABG [13,14]. If angiography of the LIMA was not perfromed and suspicion for aortoiliac disease is high, the inferior epigastric arteries may be evaluated prior to CABG using Doppler [15]. Additional studies following this screening may include abdominal aortogram, femoral arteriogram, and computed tomography angiography [16,17]. If perfusion to the lower limbs remains elusive following these angiography studies, a selective angiogram of one or both internal mammary arteries is advised [15]. Regardless of the type of diagnostic study, it is recommended to fully establish pathways of perfusion to the lower limbs in high risk patients prior to surgery [14]. If coexisting aortoiliac occlusive disease is identified preoperatively, then additional treatment options may be considered. These include performing lower extremity bypass followed by staged CABG, simultaneous CABG with lower limb revascularization, CABG utilizing saphenous vein grafts or gastroepiploic artery, and high risk multi-vessel PCI in lieu of CABG [14,18]. Restenosis or re-occlusion of coronary stents may occur, especially in the case of multiple high risk stents, which occurred in this patient. The best method to prevent occlusion of the stents is the continuation of dual antiplatelet therapy.
Given the critical condition of patients immediately following CABG, an acute ischemic limb represents a source of major morbidity and a potentially fatal insult. Additionally, given the near-routine continuation of sedation and mechanical ventilation at many facilities, ischemia, and even necrosis may go undiagnosed because of the patient's inability to report symptoms, leaving only metabolic derangements and physical exam findings to identify the issue. Although limb ischemia secondary to interruption of collateral blood flow is an important clinical consideration in Leriche syndrome, particularly if the routes of perfusion are not well identified preoperatively, the normal differential for leg ischemia following CABG must be considered. This includes but is not limited to atheromatous emboli or peripheral thrombosis, ischemia secondary to vasopressors, insufficient perfusion during cardiopulmonary bypass, and post-bypass cardiogenic shock [6].
Leriche syndrome is a diagnosis describing signs and symptoms of chronic aortoiliac occlusive disease. This syndrome has implications in the management of patients undergoing coronary angiography as well as CABG, given the routine use of the LIMA as the primary graft vessel. Aortoiliac occlusion may present challenges in gaining femoral arterial access during cardiac catheterization. Similarly, critically ill patients with this disease may be unable to benefit from intra-aortic balloon pump (IABP) therapy. The LIMA, a routine coronary artery bypass graft vessel, deserves special consideration given its common formation of collateral blood flow to the lower extremities. Perfusion of the lower extremities should be fully characterized prior to proceeding with CABG in patients with significant peripheral vascular disease or a diagnosis of aortoiliac occlusive disease [14]. Following CABG, acute postoperative lower extremity ischemia with or without necrosis may lead to loss of limb and would represent a catastrophic event in an already acutely ill patient [17].
In summary, fully determining collateral circulation prior to CABG in those patients diagnosed with or at risk for Leriche syndrome is of the utmost importance to reduce perioperative cardiac morbidity and mortality [14].
Funding
No funding was received for this manuscript or project and the authors have nothing to disclose.
References
- Patel MS, Jimenez JC, Wilson SE (2016). Aortoiliac occlusive disease: Endovascular and surgical therapies. In: Wilson SE, Jimenez JC, Veith FJ, Naylor AR, Buckels JA, editors. Vascular Surgery: Principles and Practice, 4th ed. Boca Raton: CRC Press, 2016, Chapter 16.
- Leriche R, Morel A. The Syndrome of Thrombotic Obliteration of the Aortic Bifurcation. Ann Surg. 1948; 127(2):193-206. PMID: 17859070.
- Frederick M, Newman J, Kohlwes J. Leriche Syndrome. J Gen Intern Med. 2010; 25(10):1102-4. PMID: 20568019.
- Bravković AV, Gornik I, Zlopasa O, Vrdoljak NG, Radonic R, et al. Patient with acute myocardial infarction and Leriche syndrome. Intern Med. 2010; 49(4):349-50. PMID: 20154444.
- Dietzek, AM, Goldsmith J, Veith FJ, Sanchez LA, Gupta SK, et al. Interruption of critical aortoiliac collateral circulation during nonvascular operations: a cause of acute limb-threatening ischemia. J Vasc Surg. 1990; 12(6):645-51; discussion 652-3. PMID: 2243401.
- Yurdakul M, Tola M, Ozdemir E, Bayazit M, Cumhur T. Internal thoracic artery-inferior epigastric artery as a collateral pathway in aortoiliac occlusive disease. J Vasc Surg. 2006; 43(4):707-13. PMID: 16616225.
- Akhaddar A, Eljebbouri B, Saouab R, Boucetta M. Acute paraplegia revealing Leriche syndrome. Intern Med. 2012; 51(8):981-2. PMID: 22504264.
- Lin CW, Liu CY, Chen CH. Acute renal infarction: an atypical presentation of Leriche syndrome. Intern Med. 2012; 51(17):2485. PMID: 22975574.
- McCoy CE, Patierno S, Lotfipour S. Leriche Syndrome Presenting with Multisystem Vaso-Occlusive Catastrophe. West J Emerg Med. 2015; 16(4):583-6. PMID: 26265976.
- Yoon DH, Cho H, Seol SJ, Kim T. Right calf claudication revealing leriche syndrome presenting as right sciatic neuropathy. Ann Rehabil Med. 2014; 38(1):132-7. PMID: 24639938.
- Feit A, Reddy CV, Cowley C, Ibrahim B, Zisbrod Z. Internal mammary artery angiography should be a routine component of diagnostic coronary angiography. Cathet Cardiovasc Diagn. 1992; 25(2):85-90. PMID: 1347484.
- Patel P, Shammas NW, Kapalis MJ, Dippel EJ, Lemke J, et al. Routine visualization of the left internal mammary artery before bypass surgery: is it necessary? J Invasive Cardiol. 2005; 17(9):479-81. PMID: 16145237.
- Hanet C, Marchand E, Keyeux A. Left internal mammary artery occlusion after mastectomy and radiotherapy. Am J Cardiol. 1990; 65(15):1044-5. PMID: 232734.
- Ben-Dor I, Waksman R, Satler LF, Bernardo N, Torguson R, et al. A further word of caution before using the internal mammary artery for coronary revascularization in patients with severe peripheral vascular disease! Catheter Cardiovasc Interv. 2010; 75(2):195-201. PMID: 19937782.
- Arnold, JR. Leriche's syndrome with total perfusion of the left lower extremity by way of the left internal mammary artery. Am J Cardiol. 1998; 82(8):997-9. PMID: 9794364.
- Arnold JR1, Greenberg JD, Clements S. Internal mammary artery perfusing the Leriche's syndrome. Ann Thorac Surg. 2000; 69(4):1244-6. PMID: 10800827.
- Ahmed S, Raman SP, Fishman EK. CT angiography and 3D imaging in aortoiliac occlusive disease: collateral pathways in Leriche syndrome. Abdom Radiol. 2017; 42(9):2346-2357. PMID: 28401281.
- Bobylev D, Fleissner F, Zhang R, Haverich A, Ismail I. Arterial myocardial revascularization with right internal thoracic artery and epigastric artery in a patient with Leriche's syndrome. J Cardiothorac Surg. 2013; 8:53. PMID: 23521838.