Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/094
CASE REPORT and LITERATURE REVIEW OPEN ACCESS
Case Report and Literature Review: Interventional Management of Erythromelalgia
Gregory Chinn, MD, PhD and Zhonghui Guan, MD*
Department of Anesthesia and Perioperative Care, University of California San Francisco, San Francisco, California, USA
Zhonghui Guan, MD, Associate Professor, Department of Anesthesia and Perioperative Care, University of California San Francisco, 513 Parnassus Avenue, San Francisco, CA 94117, USA, E-mail: Zhonghui.Guan@ucsf.edu
Editor: Henry Liu, MD, MS, FASA, Professor of Anesthesiology, Vice Chairman for Research, Drexel University College of Medicine, Hahnemann University Hospital, 245 N. 15th Street, MS 310, Philadelphia, PA 19102, USA, E-mail: henryliupa@gmail.com
Received: May 03, 2019 | Accepted: June 16, 2019| Published: June 29, 2019
Citation: Chinn G, Guan Z. Case Report and Literature Review: Interventional Management of Erythromelalgia. Transl Perioper & Pain Med 2019; 6(4):91-97.
Abstract
Erythromelalgia is a rare and very difficult to treat pain syndrome that usually presents as severe bilateral burning pain in the extremities. Here we present a case of a 34-year-old female with erythromelalgia who we treated successfully with a lumbar epidural infusion of ropivacaine and fentanyl. The patient had complete relief shortly after the epidural infusion, and she remained stable with only minor pain two weeks and nine months later. With this case, we have reviewed the interventional treatments of erythromelalgia. We suggest epidural infusion as the first line interventional management, followed by sympathetic block. Spinal cord stimulation can be considered if other interventional managements fail.
Introduction
Erythromelalgia is a rare pain syndrome characterized by a bilateral burning sensation in the extremities that is often aggravated by physical activity or heat. The incidence is estimated as 1.3 per 100,000 people [1]. Erythromelalgia is very difficult to treat, with only a few reported cases of complete resolution. Commonly, patients resort to soaking the affected extremities in ice water as the only effective mediator of their pain. This prolonged exposure of the painful extremities to ice water can produce secondary damage caused by the reduced circulation from the low temperature.
Erythromelalgia is classified as either primary that occurs sporadically or is inherited, or secondary that is related to various autoimmune and myeloproliferative disorders. The etiology has remained elusive until the mapping of a familial form of erythromelalgia in the SNA9a gene, which encodes the Nav1.7 sodium channel as a gain-of-function mutation resulting in hyper-excitability in dorsal root ganglion (DRG) sensory neurons [2]. Further investigation of other familial erythromelalgia lineages has resulted in more than a dozen similar gain of function mutations of Nav1.7 sodium channel [2-4]. All these mutations act to enhance the excitability of DRG neurons by lowering the firing threshold. It has been hypothesized that given the similar phenotype, the non-genetic forms of erythromelalgia may be related to DRG neuronal hyper-excitability leading to neuro-humoral changes, particularly in distal limbs. Here we present a case of erythromelalgia successfully treated with a lumbar epidural of Ropivacaine/Fentanyl infusion. We also review current interventional treatments for erythromelalgia.
Case Presentation
IRB approval was exempted from University of California San Francisco for this case report of patient treatment. A 34-year-old woman with a history of chronic constipation, recent episode of C. difficile colitis, and presumed alcoholic peripheral neuropathy presented to our medical center with severe burning pain in her feet after an exhaustive workup at an outside hospital. The pain was so severe that no medication was effective, including opioids, neuroleptics, and nonsteroidal anti-inflammatories (NSAIDs), and the only management that gave her pain relief was soaking her feet in ice baths. The pain was worse at night, but stayed at substantial severity during the daytime. She submerged her feet in ice water for 8-10 hours a day for pain relief, and she placed hot packs on her thighs to offset the cold discomfort from the ice-baths. On exam her feet were brightly erythematous and warm to touch bilaterally. Numerous ulcers were present on the dorsum of the feet (Figure 1a, and Figure 1b). She also had secondary tissue damage from both the hot packs on her thighs and the cold baths, resulting in a livedo reticularis rash (Figure 1c). She was evaluated by multiple specialists in our hospital, including neurology, vascular surgery, hospital medicine, and dermatology services, before the pain service was consulted. She had an extensive workup that included infectious, metabolic, rheumatologic, neurologic systems, with extensive imaging studies (Table 1). An electromyography (EMG) study showed an axonal neuropathy pattern. She was undergoing thiamine and folate supplementation with the thought that her pain might be related to alcohol consumption (she had periods of heavy binge drinking over the past 3 years, although none in the past 2 months).
Figure 1: Skin findings before and after epidural treatment. A,B) Images of patient's lower extremities at presentation. Note the ulcers on her feet from extended soaking in ice-water. C. Thighs have a rash consistent with livedo reticularis from heating pads to counteract the pain of soaking feet in ice-water. D-F) Feet show dramatic decrease in erythema with healthy granulation tissue forming in bed of ulcers after treatment with epidural.
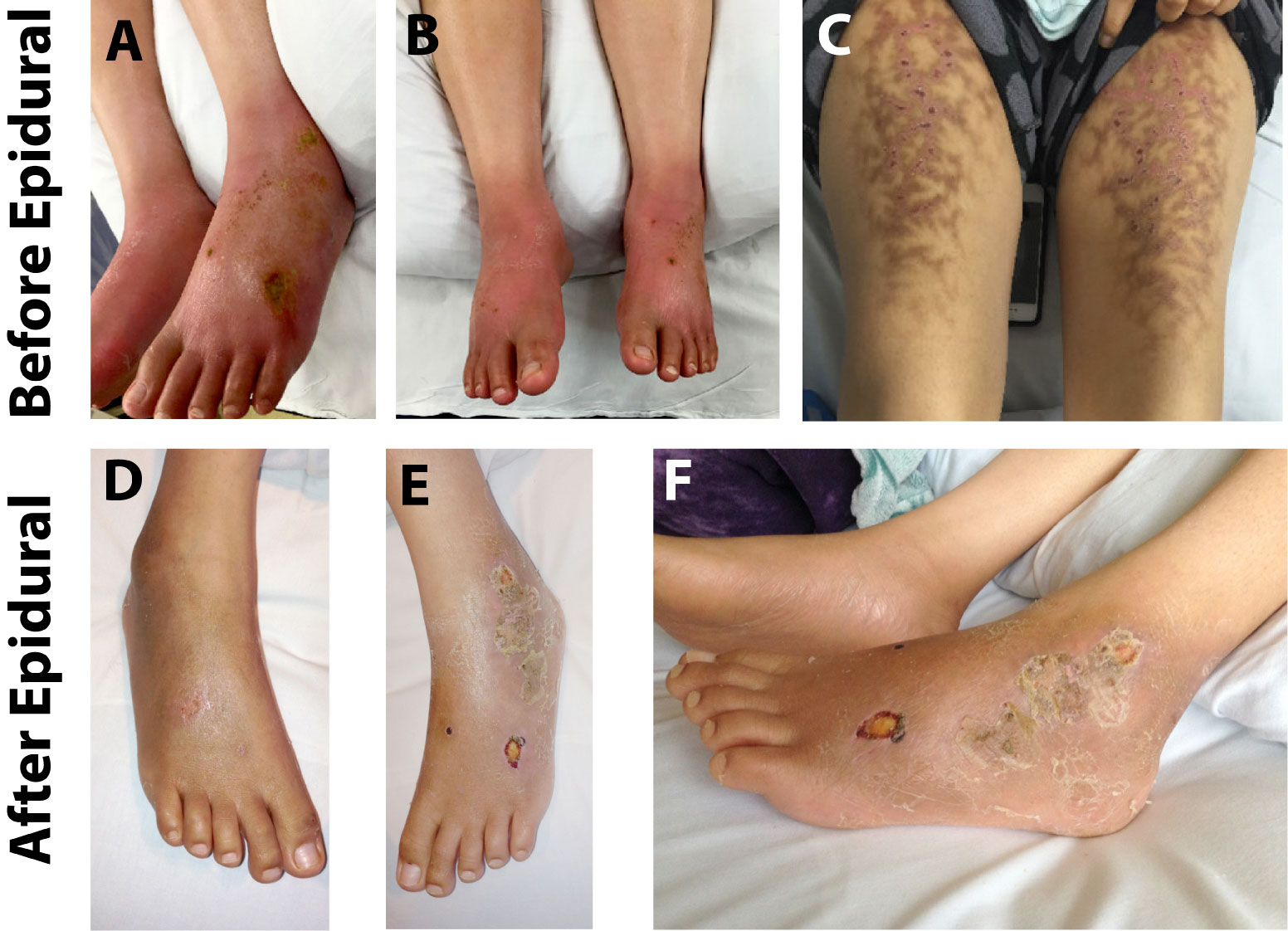
Given her classic presentation for erythromelalgia, the pain service started the full dose of aspirin because significant subpopulation of erythromelalgia patients are responsive to aspirin [5]. However, she was unresponsive to aspirin and still reported her pain as 10/10. We subsequently placed a lumbar epidural and ran a continuous infusion of 0.0625% ropivacaine with 2 mcg/ml fentanyl at 12 ml/hour. Her pain score decreased to 0-1/10 almost immediately after the initiation of epidural infusion. We continued the epidural infusion over the next three days. The patient's motor function was preserved and she was able to participate in physical therapy. On day four of epidural infusion we slowly weaned off the infusion, and the patient's pain remained at 1-2/10 after the epidural infusion was discontinued. Physical examination showed that her feet were no longer brightly erythematous, but now were darkened with pigmentation. The feet were normothermic, the allodynia had been resolved, and the chronic ulcers in her feet started to heal with healthy granulation tissue noted (Figure 1d and Figure 1f). She was discharged home the next day, and the two-week follow-up visit in our chronic pain clinic documented that her pain remained at 0-2/10, and her functional status completely returned to her baseline. At a phone follow-up at nine months, she reported that her pain was still at 0-2/10, and she was maintaining her full activities of daily living. The CURES (Controlled Substance Utilization Review and Evaluation System) report confirms that she has not used any opioid since the treatment of epidural infusion.
Discussion
Familial erythromelalgia is caused by mutation of sodium channel Nav1.7 [2] which leads to this unique constellation of symptoms. It is unlikely that our patient suffered from this genetic syndrome given her presentation in adulthood. Although we do not know the exact pathophysiology behind her symptoms, we postulate it was caused by abnormal function of DRG nociceptors with possible concomitant sympathetic nervous system dysfunction. The treatment with epidural ropivacaine served to suppress both pathways, and may have allowed a new homeostasis to occur. In addition, central sensitization in spinal cord, which could be inhibited as well by the epidural infusion of local anesthetics, might have also contributed to her pain syndrome. Whatever the mechanism, this case indicates that a standard epidural infusion should be considered as a modality to treat erythromelalgia in adults.
Medical treatment is the first line of therapies for the erythromelalgia. Commonly trialed medications include aspirin which may be effective in those with concurrent platelet dyscrasias caused by JAK-2 mutations [6]. Other commonly trialed medications include calcium channel blockers, sodium channel blockers, anticonvulsants, NSAIDs, and antidepressants [5]. However, many patients do not have a good response to medical treatment and invasive procedures for these patients may be justified.
In this case we have successfully treated an adult erythromelalgia patient with epidural infusion of 0.0625% ropivacaine with 2 mcg/ml fentanyl at 12 ml/hour for 3 days, and patient's pain was well controlled even nine months after the treatment. While there are a few reported cases of epidural treatment that have led to good pain relief in pediatric patients [7-13], to our knowledge this is one of a few adult case of erythromelalgia patient successfully managed with an epidural infusion [10,14].
Several invasive techniques have been described with varying degrees of success. Broadly they include providing sympathetic blockade or targeting central nervous system modulation. Peripheral nerve blocks, epidurals and sympathetic plexus blocks have been described with success in several cases. Although significantly different in technique, invasiveness and duration, they all likely act by inhibiting sympathetic outflow to the affected limbs. Here we summarize the relevant case reports and case series for these interventions (Table1). One hypothesis for the origin of the pain of erythromelalgia is that it is related to regional tissue hypoxia secondary to abnormal microvascular circulation. Vasodilation associated with sympathectomy is thought to improve regional blood flow and restore normoxia to affected tissues [5] which could account for the success of a variety of these techniques.
We found 8 case reports in the literature of epidural infusions to manage erythromelalgia patients from 6-year-old to 72-year-old (Table 1). Both lumbar and cervical epidural infusions have been successfully used to control pain in lower extremities and upper extremities, respectively. Epidural infusion duration varied from 2.5 days to 6 weeks, and they provided immediate and long-term (6 months to 4 years) benefits. In one case even 2 separated epidural boluses provided symptom control for over a year [14]. Although most cases, including ours, used the mixture of local anesthetic and opioids (fentanyl or morphine) for epidural treatment, epidural with local anesthetic alone without any opioid can provide similar benefit.
However, epidural is not always effective. In patients who do not respond to epidural, they may respond to sympathetic block [7]. We found 7 case reports in the literature treating erythromelalgia patients with lumbar sympathetic block from 2-month-old to 70-year-old (Table 1). Both short-term lumbar sympathetic block with local anesthetic, with or without steroid, and long-term lumbar sympathetic damage with pulse radio frequency or alcohol have been reported. No case report was found to treat erythromelalgia with stellate ganglion block, although it is very likely that stellate ganglion block might be able to provide symptom control of erythromelalgia in upper extremities.
More invasive interventional treatments like spinal cord neuromodulation has also been reported to treat erythromelalgia. There are 4 reports of trials of implanted spinal cord stimulators in patients from 15-year-old to 80-year-old [15-18] (Table 1). The leads were placed in the low thoracic region to manage symptoms of lower extremities, and in cervical region for upper extremity relief. The mechanism of action for this invasive modality is not completely understood and likely depends on the underlying cause of pain. For ischemic pain, spinal cord stimulation may inhibit sympathetic tone and improve oxygen delivery, like the sympathetic blocks, through vasodilation [19,20]. For neuropathic pain, spinal cord stimulators may relieve pain by modulating local neurotransmitter levels in the dorsal horns [19,20]. Similarly, targeting DRG neurons with stimulation has been reported as a treatment of erythromelalgia [16].
Even more invasive techniques have been described. One example is a 12-year-old with severe erythromelalgia symptoms that were leading to suicidality [21]. After infections forced the discontinuation of a spinal cord stimulation trial twice, bilateral thalamic electrodes were placed in the ventral posterolateral nuclei by a neurosurgeon. Continuous stimulation achieved improvement in pain, ablated suicidality, but had no effect on erythema. This invasive approach was inspired by an even more invasive approach described by a Soviet-era neurosurgeon of three Russian children who achieved complete resolution of erythromelalgia symptoms with stereotaxic ablation of the ventral posterolateral and the centromedian nuclei of the thalamus [22]. This irreversible, invasive, high-risk procedure performed in children raises many ethical questions, but may also offer insight into the mechanism of this disorder.
Erythromelalgia remains a challenging disorder to treat. Response to interventions will be highly dependent on the underlying cause of the disorder. Based on the risk benefit profile of all of the described invasive techniques we recommend pursuing medical therapies first, ideally to be combined with cognitive and social support that are known to improve chronic pain [23]. An epidural infusion should be considered second line as the risks are low and relief can be significant and lasting in a variety of cases. If necessary, epidural can be done at the bedside without fluoroscopy. If epidural infusion does not offer good relief, or if it is not lasting, sympathetic blockade should be considered. Local anesthetic block of the sympathetic ganglion should be trialed to assess for patient response. With a good response, neurolysis can be achieved and may offer long-lasting relief. Unlike epidural, lumbar sympathetic block requires fluoroscopy and specific procedure room. Next, we suggest a trial of spinal cord stimulator given the expense and invasive nature of spinal cord modulation. Finally, with certain cases, especially those with contraindications to spinal cord stimulator, thalamic electrode implantation may be considered. While serious risks are associated with thalamic electrode implantation, erythromelalgia in some patients can be so severe and debilitating the benefits may justify the risks.
As the genetics and pathophysiology of erythromelalgia becomes better understood, targeted therapeutics will be developed which will hopefully improve quality of life for these patients. For those who have poor response to medications, invasive techniques will continue to play a role in threating this disorder.
References
- Reed KB, Davis MDP. Incidence of erythromelalgia: a population-based study in Olmsted County, Minnesota. J Eur Acad Dermatol Venereol. 2009;23(1):13-15. doi:10.1111/j.1468-3083.2008.02938.x
- Dib-Hajj SD, Rush AM, Cummins TR, et al. Gain-of-function mutation in Nav1.7 in familial erythromelalgia induces bursting of sensory neurons. Brain. 2005;128(8):1847-1854. doi:10.1093/brain/awh514
- Dib-Hajj SD, Yang Y, Black JA, Waxman SG. The NaV1.7 sodium channel: from molecule to man. Nat Rev Neurosci. 2012;14:49.
- Waxman SG. Painful Na-channelopathies: an expanding universe. Trends Mol Med. 2013;19(7):406-409. doi:10.1016/J.MOLMED.2013.04.003
- Tham SW, Giles M. Current pain management strategies for patients with erythromelalgia: a critical review. J Pain Res. 2018;11:1689-1698. doi:10.2147/JPR.S154462
- Michiels JJ. Aspirin responsive erythromelalgia in JAK2-thrombocythemia and incurable inherited erythrothermalgia in neuropathic Nav1.7 sodium channelopathy: from Mitchell 1878 to Michiels 2017. Expert Opin Orphan Drugs. 2017;5(2):111-129. doi:10.1080/21678707.2017.1270822
- Bang YJ, Yeo JS, Kim SO, Park YH. Sympathetic Block for Treating Primary Erythromelalgia. Korean J Pain. 2010;23(1):55-59. doi:10.3344/kjp.2010.23.1.55
- D'Angelo R, Cohen IT, Brandom BW. Continuous Epidural Infusion of Bupivacaine and Fentanyl for Erythromelalgia in an Adolescent. Anesth Analg. 1992;74(1).
- Harrison CM, Goddard JM, Rittey CD. The use of regional anaesthetic blockade in a child with recurrent erythromelalgia. Arch Dis Child. 2003;88(1):65 LP - 66. doi:10.1136/adc.88.1.65
- Li X, Li Y, Qu Y, Lu L. Secondary erythromelalgia successfully treated with patient-controlled epidural analgesia and interferon α-2b: A case report and review of the literature. Exp Ther Med. 2016;11(5):1823-1826. doi:10.3892/etm.2016.3088
- Nathan A, Rose JB, Guite JW, Hehir D, Milovcich K. Primary Erythromelalgia in a Child Responding to Intravenous Lidocaine and Oral Mexiletine Treatment. Pediatrics. 2005;115(4):e504 LP-e507. doi:10.1542/peds.2004-1395
- Rauck RL, Naveira F, Speight KL, Smith BP. Refractory Idiopathic Erythromelalgia. Anesth Analg. 1996;82(5).
- Mohr M, Schneider K, Grosche M, Hildebrandt J. Zervikale epidurale Infusion von Morphin und Bupivacain bei schwerer Erythromelalgie. Anasthesiol Intensivmed Notfallmedizin Schmerztherapie. 1994;29(6):371-374. doi:10.1055/s-2007-996765
- Stricker LJ, Green CR. Resolution of refractory symptoms of secondary erythermalgia with intermittent epidural bupivacaine. Reg Anesth Pain Med. 2001;26(5):488-490. doi:10.1053/rapm.2001.25930
- Patel N, Chen E, Cucchiaro G. The Complexity of Pain Management in Patients with Erythromelalgia. A&A Pract. 2015;5(9).
- Matzke LL, Lamer TJ, Gazelka HM. Spinal Cord Stimulation for Treatment of Neuropathic Pain Associated With Erythromelalgia. Reg Anesth & Pain Med. 2016;41(5):619 LP - 620. doi:10.1097/AAP.0000000000000457
- Graziotti PJ, Goucke CR. Control of intractable pain in erythromelalgia by using spinal cord stimulation. J Pain Symptom Manage. 1993;8(7):502-504. doi:10.1016/0885-3924(93)90194-Z
- Eckmann M, Papanastassiou A, Awad M. A Unique Case for Spinal Cord Stimulation: Successful Treatment of Small Fiber Neuropathy Pain Using Multiple Spinal Cord Stimulators. Case Rep Med. 2017;2017:6969285. doi:10.1155/2017/6969285
- Oakley JC, Prager JP. Spinal Cord Stimulation: Mechanisms of Action. Spine (Phila Pa 1976). 2002;27(22).
- Linderoth B, Foreman RD. Physiology of Spinal Cord Stimulation: Review and Update. Neuromodulation Technol Neural Interface. 1999;2(3):150-164. doi:10.1046/j.1525-1403.1999.00150.x
- Delye H, Lagae L, Vermylen J, Nuttin B. Thalamic Stimulation as a Treatment for Primary Erythromelalgia: Technical Case Report. Oper Neurosurg. 2005;57(suppl_4):ONS-E404-ONS-E404. doi:10.1227/01.NEU.0000176703.27632.6D
- Kandel EI. Stereotactic Surgery of Erythromelalgia. Stereotact Funct Neurosurg. 1990;54(1-8):96-100. doi:10.1159/000100198
- Hylands-White N, Duarte R V, Raphael JH. An overview of treatment approaches for chronic pain management. Rheumatol Int. 2017;37(1):29-42. doi:10.1007/s00296-016-3481-8
- Lee CE, Paulk K, Garvie K, Grieshaber E, Carter J, Ball B. Pediatric erythromelalgia treated with epidural ropivacaine infusion. JAAD case reports. 2019;5(4):306-308. doi:10.1016/j.jdcr.2019.01.029
- Lee JY, Sim WS, Kang RA, Lee EK, Yang JY, Kim DY. Lumbar Sympathetic Pulsed Radiofrequency Treatment for Primary Erythromelalgia: A Case Report. Pediatr Dermatol. 2017;34(1):e47-e50. doi:10.1111/pde.12985
- Cerci FB, Kapural L, Yosipovitch G. Intractable erythromelalgia of the lower extremities successfully treated with lumbar sympathetic block. J Am Acad Dermatol. 2013;69(5):e270-e272. doi:10.1016/j.jaad.2013.06.047
- Galimberti D, Pontón A, Rubio L, et al. A case of primary erythromelalgia. J Eur Acad Dermatology Venereol. 2009;23(11):1338-1339. doi:10.1111/j.1468-3083.2009.03212.x
- Seishima M, Kanoh H, Izumi T, et al. A refractory case of secondary erythermalgia successfully treated with lumbar sympathetic ganglion block. Br J Dermatol. 2000;143(4):868-872. doi:10.1046/j.1365-2133.2000.03795.x
- Takeda S, Tomaru T, Higuchi M. A case of primary erythromelalgia (erythermalgia) treated with neural blockade. Japanese J Anesthesiol. 1989;38(3):383-393.
- Zoppi M, Zamponi A, Pagni E, Buoncristiano U. A way to understand erythromelalgia. J Auton Nerv Syst. 1985;13(1):85-89. doi:https://doi.org/10.1016/0165-1838(85)90008-6