Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/110
Research Article OPEN ACCESS
Long Noncoding RNA H19 in the Injured Dorsal Root Ganglion Contributes to Peripheral Nerve Injury-Induced Pain Hypersensitivity
Jing Wen1#, Yong Yang1#, Shaogen Wu1, Guihua Wei1, Shushan Jia1, Stephen Hannaford1, Yuan-Xiang Tao1,2,3*
1Department of Anesthesiology, New Jersey Medical School, Rutgers, The State University of New Jersey, Newark, NJ 07103, USA
2Department of Physiology, Pharmacology & Neuroscience, New Jersey Medical School, Rutgers, The State University of New Jersey, Newark, NJ, USA
3Department of Cell Biology & Molecular Medicine, New Jersey Medical School, Rutgers, The State University of New Jersey, Newark, NJ, USA
Dr. Yuan-Xiang Tao, Department of Anesthesiology, New Jersey Medical School, Rutgers, The State University of New Jersey, 185 S. Orange Ave., MSB, E-661, Newark, NJ 07103. Tel: +1-973-972-9812; Fax: +1-973-972-1644, E-mail: yuanxiang.tao@njms.rutgers.edu
Editor: Dr. Jun-Ming Zhang, University of Cincinnati College of Medicine, Anesthesiology, 231 Albert Sabin Way, Cincinnati, OH 45267-0531, United States, Tel: 5135582427; Email: Jun-ming.zhang@uc.edu
Received: December 18, 2019 | Accepted: January 01, 2020| Published: January 18, 2020
Citation: Wen J, Yang Y, Wu S, Wei G, Jia S, et al. Long Noncoding RNA H19 in the Injured Dorsal Root Ganglion Contributes to Peripheral Nerve Injury-Induced Pain Hypersensitivity. Transl Perioper & Pain Med 2020; 7(2):176-184.
Abstract
Peripheral nerve injury-induced changes in gene transcription and translation in the dorsal root ganglion (DRG) play a critical role in the development and maintenance of neuropathic pain. Long noncoding RNAs (lncRNAs) regulate gene expression. Here, we report that peripheral nerve injury caused by ligation of the fourth spinal nerve (SNL) led to a time-dependent increase in the expression in H19, an lncRNA, in the injured DRG. Microinjection of a specific H19 siRNA, but not negative control scrambled siRNA, into the injured DRG 4 days before SNL alleviated mechanical allodynia and thermal hyperalgesia on days 3 and 5 post-SNL. Additionally, DRG microinjection of H19 siRNA on day 7 after SNL reduced mechanical allodynia and thermal hyperalgesia on days 10 and 12 post-SNL. DRG microinjection of neither siRNA affected locomotor activity and acute basal responses to mechanical and thermal stimuli. Our findings suggest that H19 participates in the peripheral mechanism underlying the development and maintenance of neuropathic pain. H19 may be a potential target for treatment of this disorder.
Keywords
H19, Long noncoding RNA, Dorsal root ganglion, Neuropathic pain
Introduction
Neuropathic pain represents an extremely prevalent healthcare problem, afflicting millions of people, and with an annual financial impact exceeding $600 billion [1,2]. Opioids are the most widely used drugs to treat neuropathic pain, but their use is limited by a lack of efficacy during long-term use, severe side effect profiles and strong abuse liability [3,4]. It is thus imperative to identify novel targets for therapeutic treatment of neuropathic pain.
Long non-coding RNAs (lncRNAs) are transcripts more than 200 nucleotides in length that are not translated to detectable proteins. More than 15,000 lncRNAs are found in the human genome. Among these, several classes of lncRNAs have been described, including large intergenic RNA, transcribed ultraconserved regions (T-UCRs), pseudogenes, antisense RNA that overlap genes they control, and long stress-induced ncRNA [5]. They interact with DNA, proteins and other RNA, and influence transcriptional, post-transcriptional and even translational diversification of individual genes, as well as whole gene networks [6,7]. The considerable evidence indicates that lncRNAs are potent and multifunctional regulators in physiological and pathological processes, such as embryonic development, cancer, inflammation and cardiovascular and neurological diseases [8,9].
The evolutionarily conserved H19 lncRNA, as a multi-functional lncRNA, is poly-adenylated and localized predominantly in the cytoplasm. It is highly expressed in embryogenesis, and strongly down-regulated in most adult tissues [10]. Recent studies showed that H19 was identified as a powerful factor associated with many disease conditions [11-16]. Peripheral nerve injury increased the expression of H19 in the DRG [14]. This increase predominantly occurred in non-neuronal cells of DRG and Schwann cells in the injured peripheral nerve [14]. However, whether this increase contributed to neuropathic pain remains elusive. In this report, we analyzed RNA sequence data from both sham and SNL, and reported that SNL led to a marked increase in H19 in the injured DRG, which was validated by quantitative real-time reverse transcription (RT)-PCR assay. Suppression of this increase through DRG microinjection of a specific H19 siRNA into the injured DRG attenuated SNL-induced pain hypersensitivities during the development and maintenance periods. H19 likely contributed to the peripheral mechanism of neuropathic pain, and may be a potential target for therapeutic management of this disorder.
Materials and Methods
Animal preparation
Adult male CD1 mice (male, 8 weeks) were purchased from Charles River Laboratories (Raleigh, NC), and were housed in the central housing facility under a standard 12 h light/12 h dark cycle at Rutgers New Jersey Medical School, Newark, NJ. Food and water were provided ad libitum. All procedures used in this study were approved by the Animal Care and Use Committee at Rutgers New Jersey Medical School, and were consistent with ethical guidelines produced by the National Institutes of Health and the International Association for the Study of Pain. Every effort was made to minimize the number of animals used and animal suffering. Experimenters were blinded to the treatment conditions of all animals. Animals were habituated to the testing environment daily for 3 days before baseline testing. The experimenters were blinded to treatment condition during behavioral testing.
SNL-induced neuropathic pain model
The fourth lumbar (L4) spinal never ligation (SNL)-induced neuropathic pain model in mice was carried out as described previously [17-22]. Briefly, mice were anesthetized with isoflurane and the L4 spinal nerve was exposed through removal of the L4 transverse process. After exposure and isolation of the L4 spinal nerve, a tight ligation with 7-0 silk thread was made and the nerve was transected distal to the ligature. The surgical procedure for the sham group was identical to that of the SNL group, except that the spinal nerves were not transected or ligated.
DRG microinjection
H19 siRNA (Catalog number: EMU178341) was purchased from Sigma-Aldrich (St Louis, MO) and negative control (NC) siRNA (catalog number: sc-37007) from Santa Cruz Biotechnology, Inc. (Dallas, TX). TurboFect in vivo transfection reagent (Thermo Scientific Inc., Pittsburgh PA) was used as a delivery vehicle for siRNA as described [23-25] to prevent degradation and enhance cell membrane penetration of siRNA.
DRG microinjection was carried out as previously described [17-22]. Briefly, a dorsal midline incision was made in the lower lumbar region, and left L4 articular processes were exposed. The lamina was removed, and then L4 DRGs were exposed. The H19 siRNA (1 µl/DRG, 40 µM), negative control siRNA (1 µl/DRG, 40 µM), or vehicle (1 µl/DRG) was injected into the left L4 DRG with a glass micropipette connected to a Hamilton syringe for 5-10 min. The micropipette was removed 10 min after injection. The surgical field was irrigated with sterile saline and the skin incision closed with wound clips. Four days after siRNA or vehicle microinjection, SNL or sham surgery on the ipsilateral side was carried out.
Behavioral mechanical test
Paw withdrawal frequency to mechanical stimuli was carried out as described previously [17-22]. Briefly, each mouse was placed in a plastic chamber over a metal mesh floor. After 30-minute habituation, two calibrated von Frey filaments (0.07 and 0.4 g, Stoelting Co., Wool Dale, IL) were applied the plantar surface of the ipsilateral and contralateral hindpaws. Each stimulation was repeated 10 times to both hind paws. The occurrence of paw withdrawal in each of these 10 trials was expressed as a percent response frequency, and this percentage was used as an indication of the amount of paw withdrawal.
Behavioral thermal test
Paw withdrawal latency to heat stimulation was measured as described previously [17-22]. In brief, each animal was placed in a plastic chamber above a glass plate (Model 336 Analgesia Meter, IITC Life Science, Inc.). After 30-minute habituation, a radiant heat stimulus was applied by aiming a beam of light through a hole in the light box through the glass plate to the middle of the plantar surface of each hind paw. When the animal lifted its paw in response to the heat, the light beam was turned off.
The time from the start of light beam to the foot lift was defined as paw withdrawal latency. Each trial was repeated five times at 5-min intervals for each side. A cut-off time of 20 seconds was used to prevent tissue damage.
Locomotor function test
Locomotor function testing was performed as previously described [17-22]. Three reflex tests were carried out as follows. (1) Placing reflex: The mouse was held with the hind limbs slightly lower than the forelimbs, and the dorsal surfaces of the hind paws were brought into contact with the edge of a table. The experimenter recorded whether the hind paws were placed on the table surface reflexively. (2) Grasping reflex: The mouse was placed on a wire grid, and the experimenter recorded whether the hind paws grasped the wire on contact. (3) Righting reflex: The mouse was placed on its back on a flat surface, and the experimenter noted whether it immediately assumed the normal upright position. Scores for placing, grasping, and righting reflexes were based on counts of each normal reflex exhibited in five trials.
DRG cell culture and siRNA transfection
Adult (8-10 weeks) male naive mice were euthanized with isoflurane. DRGs were collected in cold DH10 [90%DMEM/F-12 (Gibco, Grand Island, NY), 10% FBS (JR Scientific, Woodland, CA), 1%penicillin-streptomycin (Quality Biological, Gaithersburg, MD)] and then treated with enzyme solution [3.5 mg/ml dispase, 1.6 mg/ml collagenase type I in HBSS without Ca2+ and Mg2+ (Gibco)] at 37℃ for 20-25 min. After centrifugation, the dissociated cells were re-suspended in DH10 and plated at a density of 1.5-4×105 cells in a 6-well plate coated with poly-L-lysine (0.5 mg/ml, Sigma, St. Louis, MO) and laminin (10 µg/ml, Invitrogen). The cells were incubated in 5% CO2 at 37℃. One day later, 500 ng siRNA was added to each well. Cells were harvested 48 hours later.
RNA extraction
The cultured DRG neurons were harvested, immediately treated in RNAlater (Ambion, Austin, TX) and stored in the freezer. Total RNA was extracted using the miRNeasy kit with on-column digestion of genomic DNA (QIAGEN, Valencia, CA) according to the manufacturer's instructions. RNA concentration was measured using the NanoDrop 2000 Spectrophotometer (Thermo Scientific, Wilmington, DE) and Qubit Fluorometric Quantitation (Invitrogen, Carlsbad, CA). Ratios of A260/280 nm were between 1.97 and 2.08.
Quantitative real-time reverse transcription (RT)-PCR
Total RNA was reverse transcribed using the ThermoScript reverse transcriptase (Invitrogen) according to the manufacturer's instructions with specific RT primers. cDNA was amplified by real-time PCR using the primers listed in Table 1 (Integrated DNA Technologies, Coralville, IA). Each sample was run in triplicate in a 20 µl reaction with 250 nM forward and reverse primers and 10 µl of Advanced Universal SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA). Reactions were performed in a BIO-RAD CFX96 real-time PCR system. The cycle parameters were set as follows: an initial 3-min incubation at 95℃, followed by 40 cycles of 95℃ for 10 s, 60℃ for 30 s, and 72℃ for 30 s. Ratios of ipsilateral-side mRNA levels to contralateral-side mRNA levels were calculated using the ΔCt method (2-ΔΔCt) at a threshold of 0.02. All data were normalized to Tubala (tubulin alpha 1 A gene), which was demonstrated to be stable after SNL in our previous studies [17,18,22,26].
Table 1: All primers used.
| Name | Sequence |
| H19-RT | 5'-AATGGGGAAACAGAGTCACG-3' |
| H19-F | 5'-CATTCTAGGCTGGGGTCAAA-3' |
| H19-R | 5'-GCCCTTCTTTTCCATTCTCC-3' |
| Kcna2-RT | 5'-GTCCCCGTCACATCTTCTCACT-3' |
| Kcna2-F | 5'-CTGCAAGGGCAACGTCACAC-3' |
| Kcna2-R | 5'-GGGACAGTGAGATGCTTGGC-3' |
| Oprm1-F | 5'-TCTTCACCCTCTGCACCATG-3' |
| Oprm1-R | 5'TCTATGGACCCCTGCCTGTA-3-' |
| Tuba1a-F | 5'-GTGCATCTCCATCCATGTTG-3' |
| Tuba1a-R | 5'-GTGGGTTCCAGGTCTACGAA-3' |
Statistical Analysis
The data were presented as the mean ± SEM. Statistical analyses were performed using one-way or two-way analysis of variance (ANOVA). If overall significance for any of the ANOVA tests was found, all pairwise multiple comparison procedures (the post-hoc Tukey-Kramer tests) were done in order to see which variables were significantly different. The criterion used for significance was p < 0.05.
Results
H19 expression is increased in the injured DRG after SNL
To determine the role of DRG H19 in neuropathic pain, we first examined whether H19 expression was changed in the injured DRG after peripheral nerve injury. We checked our previous data from next generation RNA sequencing with a higher sequencing depth, and revealed that the level of H19 was increased by 7.67-fold in the injured DRG from the SNL group compared to that from the sham group on day 7 after SNL [Sham (mean FPKM = 21.57) vs. SNL (mean FPKM = 165.44), P < 0.01] (Figure 1) [27]. To further validate the RNA sequencing data, and observe whether this increase was time-dependently associated with SNL-induced pain hypersensitivities, we carried out a combined approach of behavioral testing with quantitative real-time RT-PCR assay. In line with our previous studies [19,28-30], SNL produced mechanical allodynia as evidenced by significant increases in paw withdrawal frequencies in response to 0.07 g and 0.4 g von Frey filament stimuli (Figure 2A and Figure 2B), and thermal hyperalgesia as demonstrated by marked reductions in paw withdrawal latencies in response to heat stimulation (Figure 2C) on the ipsilateral side on days 3, 7 and 14 post-SNL. As expected, basal responses to mechanical and thermal stimuli were not altered on the contralateral side of SNL mice, or on both sides of sham mice (Figure 2A, Figure 2B and Figure 2C). Quantitative real-time RT-PCR assay revealed that SNL, but not sham surgery, led to time-dependent increases in expression of H19 in the ipsilateral L4 (injured) DRG on days 3, 7 and 14 post-SNL (Figure 2D). The ratios of ipsilateral to contralateral H19 were increased by 2.29-, 4.76- and 6.02-folds on days 3, 7 and 14 post-SNL, respectively, as compared to those at the corresponding time points after sham surgery (Figure 2D). Neither SNL nor sham surgery markedly changed basal expression of H19 in the ipsilateral L3 (intact) DRG (data not shown) and ipsilateral L4 spinal cord dorsal horn (Figure 2E). These data indicate that peripheral nerve injury leads to a time-dependent increase of H19 expression in the inured DRG, which is accompanied by pain hypersensitivities on the ipsilateral side.
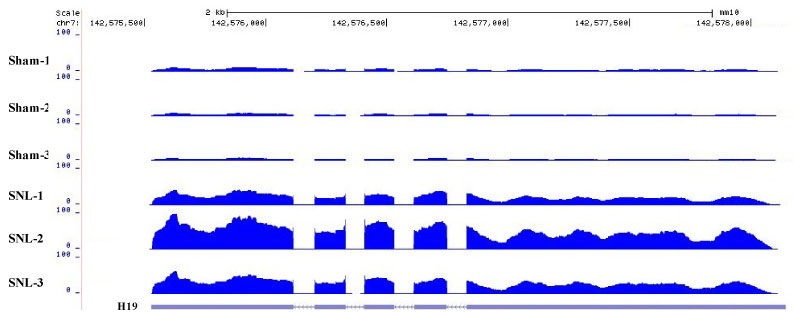
Figure 1: Visualized reads mapping to the genomic location of differentially expressed H19 gene. The stacked reads were increased in SNL samples significantly compared with sham samples in the genomic region of the H19 gene. N = 3 (30 mice) biological repeats/group.
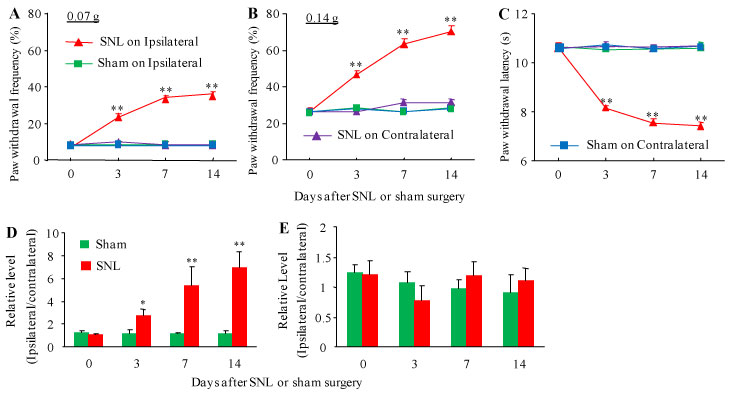
Figure 2: Peripheral nerve injury-induced time-dependent pain hypersensitivities and increase in H19 RNA in the injured DRG. (A-C) Paw withdrawal frequencies in response to 0.07 g (A) and 0.4 g (B) von Frey filament stimuli and paw withdrawal latencies in response to heat stimulation (C) on the ipsilateral and contralateral sides at the different days as indicated post-SNL or sham surgery. n = 12 mice/group. **P < 0.01 vs the sham group at the corresponding time points on the ipsilateral side. Two-way ANOVA with repeated measures followed by Tukey post hoc test. (D and E) H19 RNA expression in the ipsilateral L4 DRG (D) and ipsilateral L5 spinal cord dorsal (E) after SNL or sham surgery. Unilateral L4 DRGs from 4 mice were pooled together. n = 3 biological repeats (12 mice)/time point/group. *P < 0.05 or **P < 0.01 vs the corresponding naive mice (0 day). Two-way ANOVA with repeated measures followed by Tukey post hoc test.
Blocking increased H19 in the injured DRG alleviated the development of neuropathic pain
We further inquired whether SNL-induced increase in DRG H19 participated in the development of SNL-induced pain hypersensitivities. To this end, we blocked the SNL-induced increased of H19 in the injured DRG through pre-microinjection of H19 siRNA into the ipsilateral L4 DRGs. The H19 scrambled siRNA was used as a negative control. Selectivity and specificity of H19 siRNA were demonstrated by its ability to knockdown H19, but bot Orpm1 mRNA and Kcan2 mRNA, in in vitro transfected DRG cultured cells from adult mice (Figure 3). The amounts of H19, Orpm1 mRNA and Kcan2 mRNA in the H19 siRNA-treated group were decreased by 54%, 3% and 4.5%, respectively, as compared to those in the corresponding naive groups (Figure 3). Based on our previous reports that significant knockdown of the targeted genes via the TurboFect-mediated delivery of siRNA occurred on day 3 post-microinjection and persisted for at least 1 week [19,24-26,28,30-32], we performed SNL or sham surgery on day 4 post-microinjection. Consistent with previous studies [17-22], SNL led to long-term mechanical allodynia and thermal hyperalgesia on the ipsilateral (but not contralateral) side in the vehicle (PBS)-injected SNL mice (Figure 4A - Figure 4F). The paw withdrawal frequencies in response to 0.07 and 0.4 g von Frey filament stimuli applied to the ipsilateral hind paw were significantly increased on day 3 and 5 post-SNL, as compared to pre-injury baseline values (Figure 4A and Figure 4C). The paw withdrawal latencies of the ipsilateral hind paw in response to heat stimulation were dramatically less than those at baseline on days 3 and 5 post-SNL (Figure 4E). DRG pre-microinjection of H19 siRNA did not alter basal paw responses to mechanical and heat stimuli on the ipsilateral hind paws of sham mice (Figure 4A, Figure 4C and Figure 4E), but this pre-microinjection alleviated SNL-induced pain hypersensitivities on the ipsilateral side (Figure 4A, Figure 4C and Figure 4E). As compared to the vehicle-microinjected SNL groups at the corresponding time points, the SNL-induced increases in paw withdrawal frequencies to 0.07 g and 0.4 g von Frey filament stimuli were significantly attenuated on days 3 and 5 post-SNL, whereas the SNL-induced reductions in paw withdrawal latencies to heat stimulation were markedly blocked on days 3 and 5 post-SNL (Figure 4A, Figure 4C and Figure 4E). As expected, DRG pre-microinjection of H19 scrambled siRNA did not affect the SNL-induced pain hypersensitivities on the ipsilateral side during the observation period, as there was no marked difference in paw withdrawal frequencies or latencies between the scrambled siRNA- and vehicle-microinjected SNL groups at the corresponding time points (Figure 4A, Figure 4C and Figure 4E). DRG pre-microinjection of either H19 siRNA, scrambled siRNA, or vehicle did not alter locomotor activity on either sides (Table 2) or change basal paw frequency or latency on the contralateral side of SNL or sham mice (Figure 4B, Figure 4D and Figure 4F).
Table 2: Locomotor tests.
| Treatment groups | Placing | Grasping | Righting | |
| Vehicle + Sham | 5(0) | 5(0) | 5(0) | 5(0) |
| Vehicle + SNL | 5(0) | 5(0) | 5(0) | 5(0) |
| Scrambled siRNA + SNL | 5(0) | 5(0) | 5(0) | 5(0) |
| H19 siRNA + SNL | 5(0) | 5(0) | 5(0) | 5(0) |
| H19 siRNA + Sham | 5(0) | 5(0) | 5(0) | 5(0) |
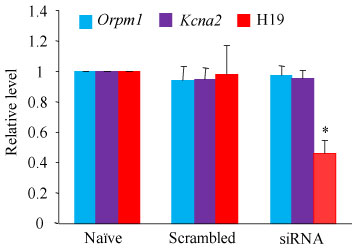
Figure 3: Effect of H19 siRNA on the expression of H19 RNA, Oprm1 mRNA and Kcan2 mRNA in the cultured DRG cells. The expression of H19 RNA, Oprm1 mRNA and Kcan2 mRNA in the cultured DRG cells from adult mice transfected without (naive) or with H19 siRNA (50 ng) or negative control scrambled siRNA (50 ng). n = 3 biological repeats/treatment. *P < 0.05 vs the corresponding naive group. One-way ANOVA with repeated measures followed by Tukey post hoc test.
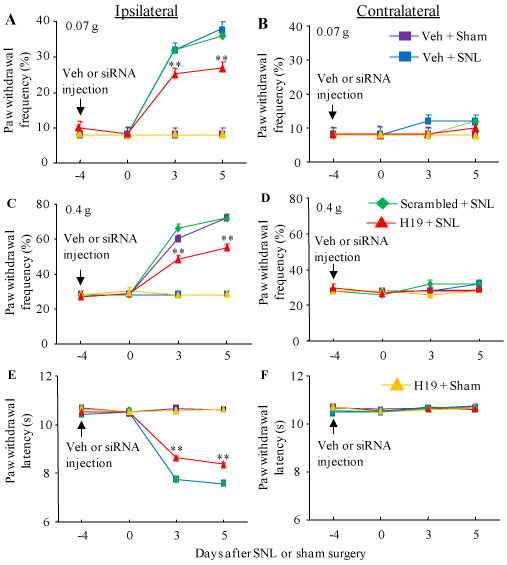
Figure 4: Effect of blocking SNL-induced increase in DRG H19 on SNL-induced development of pain hypersensitivity. SNL or sham surgery was carried out on day 4 after DRG microinjection of H19 siRNA (H19), negative control scrambled siRNA (scrambled) or vehicle (Veh). Paw withdrawal frequency to low (0.07 g; A, B) and median (0.4 g; C, D) force von Frey filament stimuli and paw withdrawal latency to thermal stimulation (E, F) at different days post-SNL or sham surgery on the ipsilateral side (A, C and E) and contralateral side (B, D and F) of mice with microinjection of H19 siRNA or negative control scrambled siRNA into the ipsilateral L4 DRG. n = 5 mice/group. ##P< 0.01 vs the vehicle-microinjected sham mice at the corresponding time point. Two-way ANOVA with repeated measures followed by post hoc Tukey test.
Blocking increased H19 in the injured DRG ameliorated the maintenance of neuropathic pain
To examine the role of DRG H19 in the maintenance of SNL-induced neuropathic pain, we microinjected vehicle or siRNA on day 7 post-SNL when SNL-induced pain hypersensitivities were robust and stable. In the vehicle-microinjected SNL mice, SNL led to mechanical allodynia as demonstrated by robust increases in paw withdrawal frequencies to 0.07g and 0.4 g von Frey filament stimuli, and thermal hyperalgesia demonstrated by significant decreases in paw withdrawal latency to heat stimulation on the ipsilateral (but not contralateral) side on days 10 and 12 post-SNL (Figure 5A- Figure F). DRG post-microinjection of H19 siRNA, but not scrambled siRNA, significantly attenuated the SNL-induced mechanical allodynia and heat hyperalgesia during the observation period (Figure 5A, Figure 5C and Figure 5E). Paw withdrawal frequencies in response to 0.07 g and 0.4 g von Frey filament stimuli decreased by 29% and 24%, respectively, on day 10 post-SNL, and by 38% and 39%, respectively, on day 12 post-SNL as compared to those in the vehicle-microinjected SNL group at the corresponding time points (Figure 5A and Figure 5C). Paw withdrawal latencies in response to heat stimuli increased by 7% and 10% on days 10 and 12, respectively, as compared to those in the vehicle-microinjected SNL group at the corresponding time points (Figure 5E). As expected, DRG post-microinjection of neither vehicle nor siRNA altered basal paw responses to mechanical and heat stimuli applied to the contralateral hind paw during the maintenance period (Figure 5B, Figure 5D and Figure 5F).
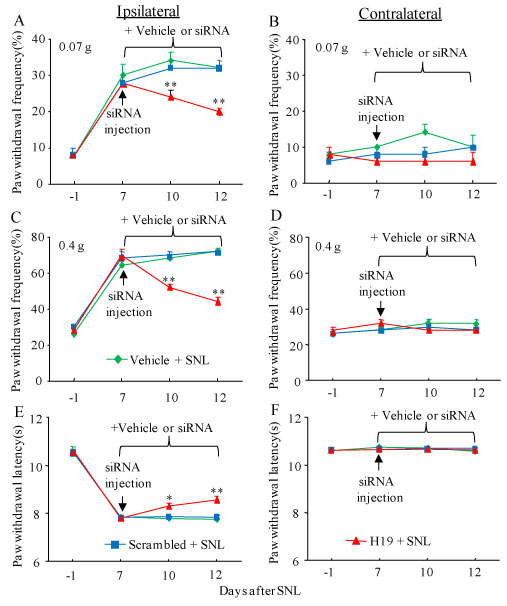
Figure 5: Effect of blocking SNL-induced increase in DRG H19 on the maintenance of SNL-induced pain hypersensitivity. Mice were subjected to SNL 7 days before DRG microinjection of H19 siRNA (H19), negative control scrambled siRNA (Scrambled) or vehicle. Paw withdrawal frequency to low (0.07 g; A and B) and medium (0.4 g; C and D) force von Frey filament stimuli and paw withdrawal latency to thermal stimulation (E and F) on both ipsilateral (A, C and E) and contralateral (B, D and F) sides of mice with microinjection of H19 siRNA, negative control scrambled siRNA or vehicle into the ipsilateral L4 DRG at different days after SNL. n = 5 mice/group. *P < 0.05 or **P < 0.01 vs the vehicle-microinjected SNL mice at the corresponding time point. Two-way ANOVA with repeated measures followed by post hoc Tukey test.
Discussion
Nerve injury-induced neuropathic pain is a complex and debilitating public health concern. Current successful treatment options for this disorder are limited [3,4,33]. Although opioids are the last option for pharmacological treatment of neuropathic pain, they cause severe side effects [34-39]. Particularly, the increase in opioid prescriptions recently in the United States has been accompanied by a huge increase in the incidence of addiction and opioid-related mortality [40]. Thus, identifying the mechanisms of neuropathic pain is essential for the discovery of novel treatments and preventative tactics for this disorder. In the present study, we demonstrated that SNL produced an increase in H19 expression in the injured DRG. Blocking this increase attenuated the development and maintenance of SNL-induced pain hypersensitivities. Our findings suggest that H19 in the DRG contributes to neuropathic pain.
H19 is widely expressed in various tissues including the brain. The amount of H19 expression is rather low in most normal adult tissues [10] and is robustly increased in response to inflammation and tissue injury under pathological conditions [11-16]. For example, circulating H19 level was significantly higher in stroke patients compared with healthy controls [16]. After middle cerebral artery occlusion in mice, H19 levels increased in plasma, white blood cells, and brain [16]. Intracerebroventricular injection of H19 siRNA reduced infarct volume and brain edema [16]. Additionally, H19 knockdown attenuated brain tissue loss and neurological deficits 14 days poststroke [16]. These results indicate that H19 plays an important role in ischemic stroke. However, whether H19 is involved in neuropathic pain was not reported previously.
The H19 gene in the DRG can be activated at the transcriptional level in response to peripheral nerve injury. A recent study reported that H19 expression was greatly increased in the ipsilateral L5 DRG of rat on days 4, 7 and 14 after SNL [14]. By in situ hybridization assay, this increase was demonstrated to occur in non-neuronal cells of the DRG [14]. Consistent with this observation, the present study further confirmed that the level of H19 was time-dependently and significantly elevated in the ipsilateral L4 DRG, but not intact L3 DRG and ipsilateral L4 dorsal horn, of mice after SNL. How peripheral nerve injury transcriptionally activates H19 gene is unclear. The potential mechanisms include the changes in epigenetic modifications and increases in transcription factors and/or RNA stability that may lead to an elevation of H19 following peripheral nerve injury. These mechanisms will be investigated in future studies.
The participation of the increased DRG H19 in neuropathic pain was demonstrated in the present study. Both pre- and post-microinjection of H19 siRNA into the injured DRG alleviated the development and maintenance of SNL-induced pain hypersensitivities. The mechanisms underlying this effect are still unknown. As discussed above, the increased H19 was observed in non-neuronal cells of the DRG after SNL [14]. This suggests that H19 siRNA microinjected into the DRG predominantly targets H19 in satellite cells and immune cells (e.g., macrophages) of the DRG. Previous study showed that H19 promoted neuroinflammation by driving HDAC1-dependent M1 microglial polarization under ischemic stroke conditions [16]. H19 also contributed to hippocampal glial cell activation via modulation of the JAK/STAT pathway during the development of epilepsy [13]. These downstream targets and/or signaling pathways likely mediate the function of DRG H19 in neuropathic pain, which will be addressed in our further studies.
In conclusion, the present study demonstrated that blocking increased H19 in the injured DRG ameliorated SNL-induced pain hypersensitivities during the development and maintenance periods, without changing acute or basal nociceptive responses and locomotor activities. Like other lncRNAs (e.g., Kcna2 antisense RNA) [22,41,42], H19 may be a potential target for therapeutic treatment of neuropathic pain. However, given that majority of body tissues express H19, potential unwanted effects caused by targeting H19 should be considered.
Acknowledgments
This work was supported by NIH grants (R01NS094664, R01NS094224, R01NS111553 and RFNS113881) to Y.X.T.
Author Contributions
Y.X.T. conceived the project and supervised all experiments. Y.Y., J.W., S.W. and Y.X.T. assisted with experimental design. Y.Y., J.W., S.W., G.W. and S.J. carried out animal surgery and molecular, biochemical, morphological, and behavioral experiments. Y.Y., J.W., S.W., S.H. and Y.X.T. analyzed the data. Y.X.T. wrote the draft of the manuscript. All authors read and discussed the manuscript.
Competing Financial Interests
The authors declare no competing financial interests.
References
- DiBonaventura MD, Sadosky A, Concialdi K, Hopps M, Kudel I, Parsons B et al. The prevalence of probable neuropathic pain in the US: results from a multimodal general-population health survey. J Pain Res 2017; 10: 2525-38.
- van HO, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 2014; 155(4): 654-62.
- Boudreau D, Von KM, Rutter CM, Saunders K, Ray GT, Sullivan MD et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf 2009; 18(12): 1166-75.
- Toblin RL, Mack KA, Perveen G, Paulozzi LJ. A population-based survey of chronic pain and its treatment with prescription drugs. Pain 2011; 152(6): 1249-55.
- Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell 2013; 152(6): 1298-307.
- Qureshi IA, Mehler MF. Emerging roles of non-coding RNAs in brain evolution, development, plasticity and disease. Nat Rev Neurosci 2012; 13(8): 528-41.
- Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell 2011; 43(6): 904-14.
- Lekka E, Hall J. Noncoding RNAs in disease. FEBS Lett 2018; 592(17): 2884-900.
- Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms. Cell 2013; 154(1): 26-46.
- Gabory A, Jammes H, Dandolo L. The H19 locus: role of an imprinted non-coding RNA in growth and development. Bioessays 2010; 32(6): 473-80.
- Cao T, Jiang Y, Wang Z, Zhang N, Al-Hendy A, Mamillapalli R et al. H19 lncRNA identified as a master regulator of genes that drive uterine leiomyomas. Oncogene 2019; 38(27): 5356-66.
- Ghazal S, McKinnon B, Zhou J, Mueller M, Men Y, Yang L et al. H19 lncRNA alters stromal cell growth via IGF signaling in the endometrium of women with endometriosis. EMBO Mol Med 2015; 7(8): 996-1003.
- Han CL, Ge M, Liu YP, Zhao XM, Wang KL, Chen N et al. LncRNA H19 contributes to hippocampal glial cell activation via JAK/STAT signaling in a rat model of temporal lobe epilepsy. J Neuroinflammation 2018; 15(1): 103.
- Iwasaki H, Sakai A, Maruyama M, Ito T, Sakamoto A, Suzuki H. Increased H19 Long Non-coding RNA Expression in Schwann Cells in Peripheral Neuropathic Pain. J Nippon Med Sch 2019; 86(4): 215-21.
- Peng L, Yuan XQ, Liu ZY, Li WL, Zhang CY, Zhang YQ et al. High lncRNA H19 expression as prognostic indicator: data mining in female cancers and polling analysis in non-female cancers. Oncotarget 2017; 8(1): 1655-67.
- Wang J, Zhao H, Fan Z, Li G, Ma Q, Tao Z et al. Long Noncoding RNA H19 Promotes Neuroinflammation in Ischemic Stroke by Driving Histone Deacetylase 1-Dependent M1 Microglial Polarization. Stroke 2017; 48(8): 2211-21.
- Liang L, Gu X, Zhao JY, Wu S, Miao X, Xiao J et al. G9a participates in nerve injury-induced Kcna2 downregulation in primary sensory neurons. Sci Rep 2016; 6: 37704.
- Liang L, Zhao JY, Gu X, Wu S, Mo K, Xiong M et al. G9a inhibits CREB-triggered expression of mu opioid receptor in primary sensory neurons following peripheral nerve injury. Mol Pain 2016; 12: 1-16.
- Mo K, Wu S, Gu X, Xiong M, Cai W, Atianjoh FE et al. MBD1 Contributes to the Genesis of Acute Pain and Neuropathic Pain by Epigenetic Silencing of Oprm1 and Kcna2 Genes in Primary Sensory Neurons. J Neurosci 2018; 38(46): 9883-99.
- Sun L, Zhao JY, Gu X, Liang L, Wu S, Mo K et al. Nerve injury-induced epigenetic silencing of opioid receptors controlled by DNMT3a in primary afferent neurons. Pain 2017; 158(6): 1153-65.
- Zhao JY, Liang L, Gu X, Li Z, Wu S, Sun L et al. DNA methyltransferase DNMT3a contributes to neuropathic pain by repressing Kcna2 in primary afferent neurons. Nat Commun 2017; 8: 14712.
- Zhao X, Tang Z, Zhang H, Atianjoh FE, Zhao JY, Liang L et al. A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat Neurosci 2013; 16(8): 1024-31.
- Li Z, Gu X, Sun L, Wu S, Liang L, Cao J et al. Dorsal root ganglion myeloid zinc finger protein 1 contributes to neuropathic pain after peripheral nerve trauma. Pain 2015; 156(4): 711-21.
- Xu JT, Zhou X, Zhao X, Ligons D, Tiwari V, Lee CY et al. Opioid receptor-triggered spinal mTORC1 activation contributes to morphine tolerance and hyperalgesia. J Clin Invest 2014; 124: 592-603.
- Zhang J, Liang L, Miao X, Wu S, Cao J, Tao B et al. Contribution of the Suppressor of Variegation 3-9 Homolog 1 in Dorsal Root Ganglia and Spinal Cord Dorsal Horn to Nerve Injury-induced Nociceptive Hypersensitivity. Anesthesiology 2016; 125(4): 765-78.
- Mao Q, Wu S, Gu X, Du S, Mo K, Sun L et al. DNMT3a-triggered downregulation of K2p 1.1 gene in primary sensory neurons contributes to paclitaxel-induced neuropathic pain. Int J Cancer 2019.
- Wu S, Marie LB, Miao X, Liang L, Mo K, Chang YJ et al. Dorsal root ganglion transcriptome analysis following peripheral nerve injury in mice. Mol Pain 2016; 12: 1-14.
- Huang LN, Zou Y, Wu SG, Zhang HH, Mao QX, Li JB, Tao YX. Fn14 Participates in Neuropathic Pain Through NF-kappaB Pathway in Primary Sensory Neurons. Mol Neurobiol 2019; 56(10): 7085-96.
- Wu Q, Wei G, Ji F, Jia S, Wu S, Guo X et al. TET1 Overexpression Mitigates Neuropathic Pain Through Rescuing the Expression of mu-Opioid Receptor and Kv1.2 in the Primary Sensory Neurons. Neurotherapeutics 2019; 16(2): 491-504.
- Xu B, Cao J, Zhang J, Jia S, Wu S, Mo K et al. Role of MicroRNA-143 in Nerve Injury-Induced Upregulation of Dnmt3a Expression in Primary Sensory Neurons. Front Mol Neurosci 2017; 10: 350.
- Li Z, Mao Y, Liang L, Wu S, Yuan J, Mo K et al. The transcription factor C/EBPbeta in the dorsal root ganglion contributes to peripheral nerve trauma-induced nociceptive hypersensitivity. Sci Signal 2017; 10(487): eaam5345.
- Yuan J, Wen J, Wu S, Mao Y, Mo K, Li Z et al. Contribution of dorsal root ganglion octamer transcription factor 1 to neuropathic pain after peripheral nerve injury. Pain 2019; 160(2): 375-84.
- Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron 2006; 52(1): 77-92.
- Obara I, Przewlocki R, Przewlocka B. Local peripheral effects of mu-opioid receptor agonists in neuropathic pain in rats. Neurosci Lett 2004; 360(1-2): 85-9.
- Obara I, Makuch W, Spetea M, Schutz J, Schmidhammer H, Przewlocki R, Przewlocka B. Local peripheral antinociceptive effects of 14-O-methyloxymorphone derivatives in inflammatory and neuropathic pain in the rat. Eur J Pharmacol 2007; 558(1-3): 60-7.
- Obara I, Parkitna JR, Korostynski M, Makuch W, Kaminska D, Przewlocka B, Przewlocki R. Local peripheral opioid effects and expression of opioid genes in the spinal cord and dorsal root ganglia in neuropathic and inflammatory pain. Pain 2009; 141(3): 283-91.
- Rashid MH, Inoue M, Toda K, Ueda H. Loss of peripheral morphine analgesia contributes to the reduced effectiveness of systemic morphine in neuropathic pain. J Pharmacol Exp Ther 2004; 309(1): 380-7.
- Zhou XL, Yu LN, Wang Y, Tang LH, Peng YN, Cao JL, Yan M. Increased methylation of the MOR gene proximal promoter in primary sensory neurons plays a crucial role in the decreased analgesic effect of opioids in neuropathic pain. Mol Pain 2014; 10: 51.
- Uchida H, Ma L, Ueda H. Epigenetic gene silencing underlies C-fiber dysfunctions in neuropathic pain. J Neurosci 2010; 30(13): 4806-14.
- Meyer R, Patel AM, Rattana SK, Quock TP, Mody SH. Prescription opioid abuse: a literature review of the clinical and economic burden in the United States. Popul Health Manag 2014; 17(6): 372-87.
- Fan L, Guan X, Wang W, Zhao JY, Zhang H, Tiwari V et al. Impaired neuropathic pain and preserved acute pain in rats overexpressing voltage-gated potassium channel subunit Kv1.2 in primary afferent neurons. Mol Pain 2014; 10(1): 8.
- Wu S, Bono J, Tao YX. Long noncoding RNA (lncRNA): A target in neuropathic pain. Expert Opin Ther Targets 2018.