Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/119
Review Article OPEN ACCESS
Remote Ischemic Preconditioning for Cardioprotection in Patients Undergoing Cardiac Surgery: A Systemic Review
Sheng Wang1,2*, Xiaodong Ye3, Jinfeng Wei1 and Zhengyuan Xia3,4
1Department of Anesthesiology, Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou, Guangdong Province, China
2Department of Anesthesiology, Linzhi People's Hospital, Linzhi, Tibet, China
3Department of Anesthesiology, The University of Hong Kong, Hong Kong SAR, China
4Department of Anesthesiology, Affiliated Hospital of Guangdong Medical University, Zhanjiang, China
Dr. Sheng Wang, Department of Anesthesiology, Guangdong Cardiovascular Institute & Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou, Guangdong Province, China, E-mail: shangwang_gz@163.com
Editor: Renyu Liu, MD, PhD, Professor, Department of Anesthesiology and Critical Care, Perelman School of Medicine at the University of Pennsylvania, Center of Penn Global Health Scholar, Director of Stroke 120 Special Task Force, Chinese Stroke Association, 336 John Morgan building, 3620 Hamilton Walk, Philadelphia, PA 19104, USA, Phone: 2157461485, Fax: 2153495078, E-mail: RenYu.Liu@pennmedicine.upenn.edu
Received: March 07, 2020 | Accepted: March 13, 2020 | Published: March 20, 2020
Citation: Wang S, Ye X, Wei J, Xia Z. Remote Ischemic Preconditioning for Cardioprotection in Patients Undergoing Cardiac Surgery: A Systemic Review. Transl Perioper & Pain Med 2020; 7(3):238-247
Abstract
A short period of sublethal ischemia can provide cardioprotection against ischemia/reperfusion injury. As direct application of ischemic to the heart is impractical, remote ischemic preconditioning (RIPC) is usually preferred. The mechanism through which the cardioprotective effect is transferred from the remote organ or tissue to the heart remains unclear. Neurogenic, hormonal, and systemic factors have been proposed. Although RIPC has been applied in clinic with different modes, its effect on major cardiac outcomes, especially among diabetic patients, is still controversial. Therefore, further studies are still needed to identify its exact role in clinical practice and also investigate more specific mechanisms.
Myocardial Ischemia and Reperfusion Injury
Myocardial ischemia is a major cause of myocardial injury and may result in myocardial infarction. Reperfusion therapies or interventions to restore blood flow to ischemic myocardium are needed to limit infarct size, but, return of blood flow can cause additional cardiac damage and complications which is known as reperfusion injury. Nevertheless, myocardium possesses adaptive mechanisms which can resist potentially lethal Ischemia and Reperfusion Injury.
Ischemic preconditioning (IPC), a short period of sublethal ischemia intervention prior to a prolonged ischemic event, was discovered over 30 years ago by Reimer and Murry, et al. [1,2] and was believed to be capable of providing myocardial protection against ischemia/reperfusion (I/R) injury. Although IPC is a reproducible cardioprotective intervention [3], it has not been fully translated into clinical practice. The major limitation of myocardial IPC is that it requires a protective stimulus to be applied to the heart directly, which is usually invasive with unpredictable adverse effect [4] and thus infeasible.
Accordingly, remote ischemic preconditioning (RIPC) has been considered as more feasible and promising than IPC due to its non-invasiveness. RIPC utilizes transient ischemia and reperfusion of one vascular bed, organ or tissue away from the heart to precondition another vascular bed. It has been applied before the onset of sustained myocardial ischemia [5]. However, the effect of RIPC on clinical outcome among different patients is controversial [6-8]. The differences and complications of patient population involved or differences in the RIPC procedures or the timing of application in different studies may be possible explanations for the controversies observed. However, the major reason for the uncertainty of RIPC cardiopropection is likely the lack of a clear understanding of the mechanism RIPC.
Definition and differences of Conditionings IPC, RIPC and APC that are Applicable During Cardiac Surgery
Ischemic preconditioning (IPC), defined as several brief periods of ischemia and reperfusion of the target organ before prolonged ischemia [9], is regarded as a protective means capable of resisting myocardial ischemia-reperfusion injury (IRI). During cardiac surgery such as coronary artery bypass surgery (CABG), coronary artery is inaccessible for IPC [9]. The alternative to IPC is remote ischemic precondition (RIPC), a noninvasive simple method, which is induced by several rounds of short-term remote organ (e.g. upper limbs) ischemia and reperfusion before prolonged myocardial ischemia [6,10,11]. There have been trials confirming that RIPC can significantly reduce cardiac specific enzymes troponin I (cTnI) [10] and cTnT [11] levels in the serum within 72 hours after surgery, as well as decrease myocardium infarct size [7] and promote post-operative patient recovery [12]. However, whether or not it can improve long term cardiovascular outcomes still remains controversial [7,13], despite that the beneficial effect of RIPC on new onset atrial fibrillation post-cardiac surgery seems to be more confirmative [14]. In the meantime, there is also a growing interest in understanding non-anesthetic effects of narcotics [15]. For example, anesthetic pre/postconditioning (APC) is considered to have a positive effect on protecting ischemic organs, which is analogous to IPC [9,15]. APC is achieved by using anesthetic (e.g. sevoflurane, isoflurane, propofol) for a brief period before and after target organ ischemic exposure [15,16-18]. However, the exact protective effect and possible adverse outcomes of using extra amount of anesthetics before the operation are still need to be explored.
Potential Mechanism of RIPC
The potential mechanisms underlying remote ischemic preconditioning (RIPC) are still not fully understood. A better mechanistic understanding will help translate its cardioprotective use to clinical practice. Currently, it has been suggested that the mechanism of RIPC may be based on three steps: Stimuli at remote sites, protective signal transferred through neural or humoral pathways to the target organ, leading to the final activation of receptors and intracellular signal transduction pathways as illustrated in Figure 1.
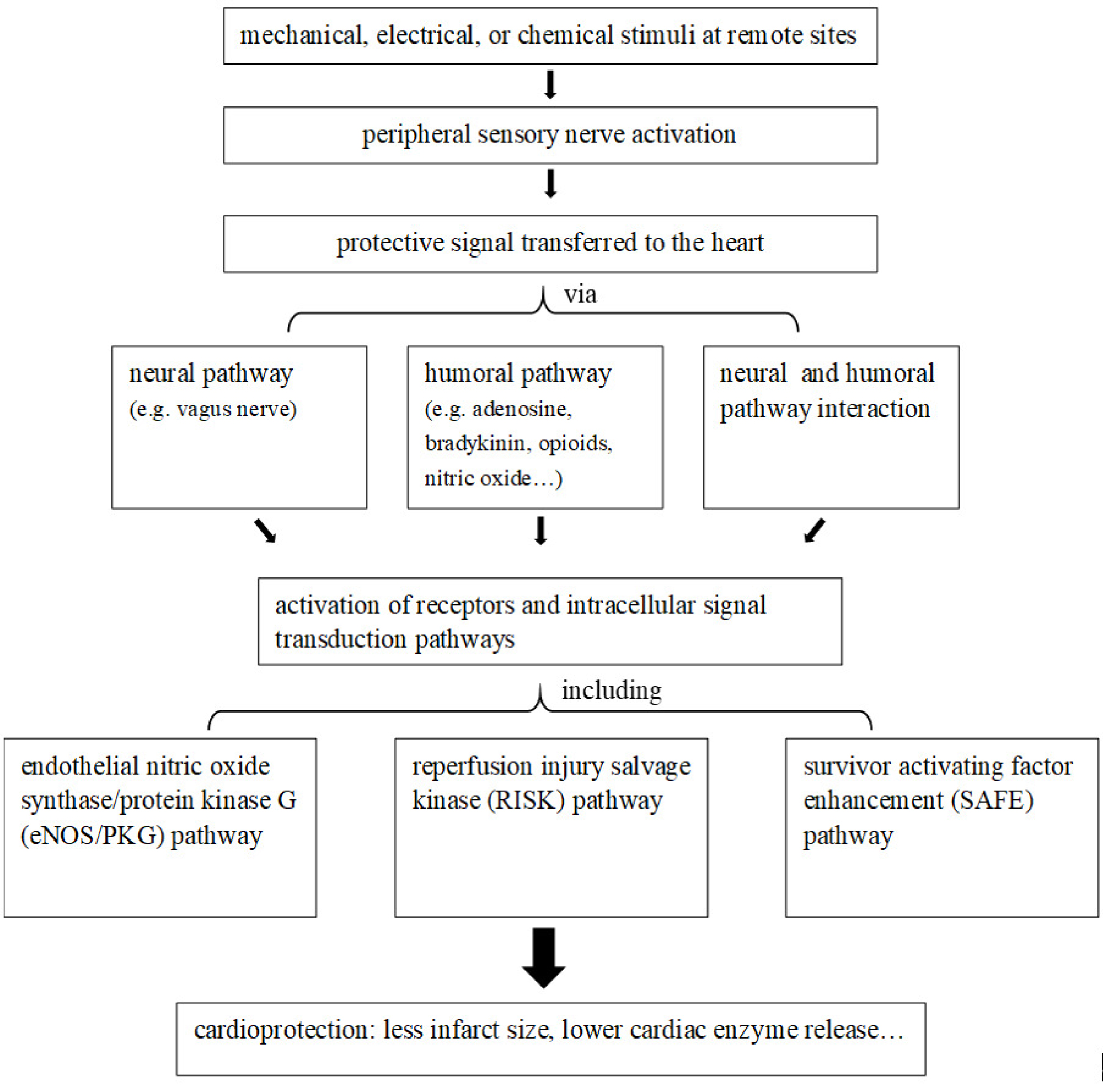
Figure 1: Diagram of possible mechanisms/pathways of remote ischemic preconditioning cardioprotection.
The stimuli that act on the remote sites to activate the peripheral sensory nerves can be electrical [19], chemical, mechanical [20] or ischemic [21,22]. Studies have demonstrated that the transfer of the protective signal from remote stimulus site to the heart involves neural pathway, humoral pathway, or even their interaction [23]. It has been suggested that the vagus nerve might be the link between sensory nerve activation in the limb and release of the humoral mediator [24]. Experiments using pigs and rats showed that a vago-splenic axis was involved in the cardioprotection of RIPC [25]. Substances such as adenosine [26], bradykinin, opioids [27], microvesicles [28], microRNA-144 [29], nitric oxide [30,31], interleukin (IL)-10 [32] and stromal cell-derived factor-1a [33] have been suggested to be the humoral factors of RIPC. Experimentally, RIPC has been shown to induce exosomes release into the plasma to transfer microRNA -24 in a paracrine manner and that microRNA -24 in the exosomes plays a central role in mediating the protective effects of RIPC [34]. However, whether or not RIPC may induce exosomes to transfer microRNAs confer cardioprotection in the patients undergoing cardiac surgery has yet to be explored.
When autacoids and neurohormones are circulated to target cells via the blood stream, they activate receptors and further initiate intracellular signal transduction. Three main intracellular signal transduction pathways include the endothelial nitric oxide synthase/protein kinase G (eNOS/PKG) pathway, the reperfusion injury salvage kinase (RISK) pathway and the survivor activating factor enhancement (SAFE) pathway [35]. RIPC reduced myocardial infarct size with the activation of the PI3K-Akt pathway at reperfusion in the porcine heart [36]. Experimental evidence supported that Stat5 was also involved in RIPC and played an important role through anti-apoptotic signaling and also PI3K/AKT survival pathway [37]. Ultimately, mitochondria, the major cellular ATP source, have been identified as potential effectors in cardioprotection [32]. However, more details of the RIPC mechanism still need to be explored.
Debate on Clinical Trials of RIPC (Negative/Positive)
We searched literature in Pubmed and the searching result is summarized in Figure 2 and Table 1.
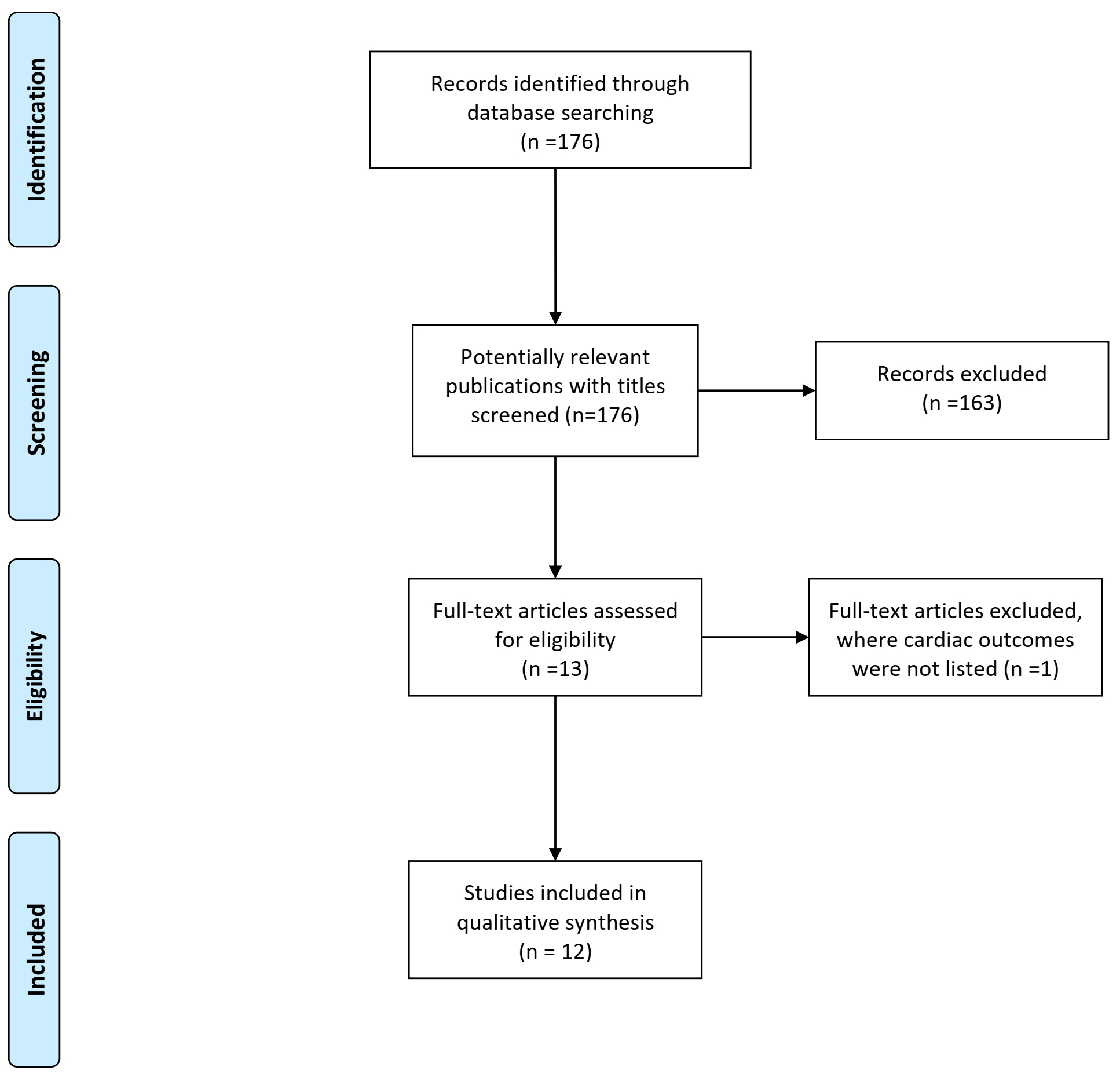
Figure 2: Literature searching results.
Table 1: Summary of RIPC clinical trials.
| Ischemia method | Duration | I/R Cycles | Surgery | Prognosis | |
| Stephen P. Hoole | Upper limb | 5 minutes | 3 | PCI | Improved |
| Qingping Wu | Lower limb | 5 minutes | 3 | TOF | Improved |
| Rianne Nederlof | Upper limb | 5 minutes | 3 | CABG | No effect |
| Brian W. McCrindle | Left thigh | 5 minutes | 4 | Cardiac surgery | No effect |
| Derek J Hausenloy | Upper limb | 5 minutes | 4 | PCI | No effect |
| Stewart R. Walsh | Lower limb | 10 minutes | 2 | Carotid endarterectomy | No effect |
| Z. Cao | Lower limb | 5 minutes | 3 | Cardiac surgery | No effect |
| Ruijuan Han | Upper limb | 5 minutes | 4 | Radiofrequency ablation | Better |
| Nicole S. Coverdale | Upper limb | 5 minutes | 3 | Cardiac surgery | No effect |
| Patrick Meybohm | Upper limb | 5 minutes | 4 | Cardiac surgery | No effect |
| D.J. Hausenloy | Upper limb | 5 minutes | 4 | CABG | No effect |
| P. Meybohm | Upper limb | 5 minutes | 4 | Cardiac surgery | No effect |
Some meta-analysis studies [38-40] has demonstrated that RIPC can reduce the periprocedural myocardial infarctions (PMIs) in patients undergoing elective PCI. Davies, et al. [41], also reported a lower rate of major adverse cardiac and cerebral event (MACCE) in RIPC groups after 6 years. While Prasad, et al. [42], and Hausenloy, et al. [7], found no improvement in 1-year follow-up outcomes. The heterogeneity of the study protocols might be the reason for different results of the trials. For example, RIPC cycles were not exactly the same and the percentage of patients with peripheral vascular diseases were also different. On the other hand, the unpredictability of angina presents a challenge to the use of RIPC. The coronary collateral blood flow (CCBF) to the infarct-related artery seems to be more important for the cardioprotective efficacy of RIPC [43].
Elective coronary artery bypass surgery (CABG) stands as an alternative to elective PCI. A randomized controlled study which enrolled 180 adult patients undergoing elective CABG surgery [44] showed a significant decrease of serum high sensitive troponin T and acute kidney injury in RIPC group. Thielmann, et al. [10], drew a similar conclusion. Qingping, et al. [45] demonstrated that RIPC attenuates myocardial IRI and improves the short-term prognosis of pediatric patients with tetralogy of fallot (TOF) underwent open heart surgery. Some other randomized trials also confirmed a cardioprotective role of RIPC [46] . On the contrary, a recent meta-analysis study [47] found negative evidence for the beneficial effects of RIPC. A randomized trial [48] enrolling 1612 patients also demonstrated a negative result. The key influencing factors might include the lack of standardized anaesthetic management and sub-group analysis, which could reduce the power of statistical analysis. In all, RIPC seems to have a protective role during PMI. Yet more standardized protocols and effectiveness studies are needed to reveal the exact role of RIPC.
Modes of RIPC and its Evolution
Remote ischemic preconditioning (RIPC)was found to be repeatable by simple inflation-deflation of the cuff around the limbs, which was a turning point in the clinical application of cardiac protection [49]. In 2000, the first small clinical study that included four patients in each group undergoing CABG of RIPC didn't show any difference in the CK-MB levels [50]. However, in 2006, a clinical trial of RIPC in pediatric patients undergoing congenital heart surgery demonstrated significant reduction in cardiac enzyme release, which was the first study to reveal that RIPC provided perioperative myocardial protection in humans [51]. Most subsequent studies focused on cardiac injury bio-markers, which were both consistent and reasonable. With increasing supportive evidence, RIPC might alter the short and long term prognosis of patients after cardiac surgery [52]. The effect of RIPC has already been widely investigated in coronary artery bypass graft (CABG) surgery, cardiac valvular surgery, percutaneous coronary intervention (PCI), and major vascular surgery. However, current clinical evidence for RIPC cardioprotection is not convincing enough.
A recent study of RIPC in 65 patients undergoing off pump CABG surgery showed that RIPC by four episodes of 5-min inflation-deflation of the cuff around the upper limb shortened mechanical ventilation time [11]. Meanwhile, a single-center randomized, double-blind, controlled trial of RIPC in 329 patients undergoing elective isolated first-time CABG surgery under cold crystalloid cardioplegia and cardiopulmonary bypass showed that RIPC reduced perioperative myocardial injury during elective CABG surgery with better survival and lower incidence of major adverse cardiac events [6]. However, the Cochrane systematic review of 5392 patients undergoing CABG with or without valvular surgery showed that RIPC couldn't provide any clinical benefit [47].
Furthermore, a systematic meta-analysis of 2,200 patients undergoing cardiovascular surgery demonstrated no significant benefits from perioperative outcomes [53]. Age, anesthesia and comorbidities that induce fundamental alternations of cellular signaling cascades may attenuate the beneficial effect of RIPC [54]. Cardioprotective therapy with beta-blockers or statins may also influence the clinical effect of RIPC [55]. Another systematic meta-analysis of 5262 patients undergoing cardiac surgery concluded that RIPC didn't reduce the morbidity or mortality in patients undergoing cardiac surgery with cardiopulmonary bypass. In subgroup studies without propofol, acute kidney injury (AKI) incidence was reduced, indicating that propofol may interact with the RIPC of cardiac protection [56].
Some studies investigated the role of RIPC in emergency and elective PCI and concluded with different results. Davies, et al., concluded that major adverse cardiac and cerebral events were significantly reduced in patients with RIPC after PCI [41]. RIPC combined with PCI increased myocardial salvage before hospitalization in a randomized trial [6]. The RIC-STEMI trial by Gaspar demonstrated that improved clinical outcomes were found with RIPC after a median follow-up of 2.1 years without any reduction in the size of myocardial infarct [57]. A meta-analysis of 16 randomized trials showed that RIPC, using inflation-deflation of the cuff around the limbs, provided cardiac and renal protection for patients undergoing elective PCI [58]. However, an international randomized controlled trial including 33 centers across the UK, Denmark, Spain, and Serbia enrolling patients (age >18 years) with suspected STEMI and also receiving RIPC treatment before primary percutaneous coronary intervention (PPCI) showed that RIPC did not improve postoperative prognosis at 12 months in patients with STEMI undergoing PPCI, neither had beneficial effect on myocardial infarct size [7].
Studies of RIPC in major vascular surgery, including open abdominal aortic aneurysm repair (AAAR) and endovascular aneurysm repair (EVAR), were also evaluated. Ali, et al., found a significant reduction in myocardial infarction and renal injury with the RIPC intervention where iliac artery cross clamp was used as the preconditioning stimulus after AAAR [59], while Walsh, et al., could not demonstrate improved outcome with the same intervention [60]. Acute lower limb ischemia requiring operation was found in 4 patients, suggesting elevated concerns about the appropriateness of iliac artery cross clamp as the preconditioning stimulus. The study of patients undergoing EVAR demonstrated no significance in renal protection or other clinical benefits [61].
Impacts of Diabetes on the Effectiveness of RIPC in Cardiac Surgery
It has been reported that RIPC could be a potential protective approach for perioperative complications. RIPC could reduce myocardial injury by decreasing oxidative stress, reduce productions of inflammatory cytokines, activate protective signaling pathways, and also promote the release of neuroendocrine factors [11,62]. However, the beneficial effect of RIPC in diabetic subjects has always been very controversial.
It has been reported that diabetic patients are more sensitive to ischemia-reperfusion injury (IRI), though the mechanism is still not fully understood. Alterations in cardiac metabolism have been suggested to play a key role in its pathophysiology. There are significant changes in energy metabolism in diabetic patients. The utilization of glucose decreases, and the heart switches from glucose utilization to predominantly fatty acids (FA) uptake for energy supply, which subsequently increases FA oxidation and triglyceride (TG) accumulation in the heart [63]. In diabetic heart, high levels of FA delivery exceeds the oxidative capacity of the cell, resulting in greater oxygen demand, mitochondrial uncoupling, reactive oxygen species (ROS) overproduction, and mitochondrial dysfunction, which would then lead to cardiomyocyte death and finally exacerbate myocardial IRI [64]. This might explain why many studies couldn't yield a positive effect of RIPC on diabetes [9].
Moreover, diabetes is proposed to adversely affect the cardiac pro-survival signalings such as the STAT3 signaling [65,66] to abolish cardioprotective effect of RIPC [21,67] . Diabetes hampers the development of cardioprotective response to RIPC by impairing the activation of cardioprotective signaling pathways, like the impairment in O-linked β-N-acetyl glucosamine (O-GlcNAc) signaling and release of cardioprotective humoral factors may contribute to attenuating RIPC-induced cardioprotection [68].
Neural pathway is also involved in mediating the cardioprotective effects of RIPC. There is a release of a cardioprotective humoral factor during RIPC. However, the release of the cardioprotective factor is dependent on the intact neural pathway [69]. Lim SY, et al., found that resection of femoral or the sciatic nerves partially blocked RIPC-induced cardioprotection, while resection of both nerves completely abolished the cardioprotective effect of RIPC [23]. Peripheral neuropathy is common in patients with diabetes, especially those with long-standing diabetes. Therefore, the release of cardioprotective factor may be inhibited among diabetic patients, thus attenuating the protective effect of RICP on myocardial ischemia-reperfusion injury.
O-GlcNAc is an intercellular carbohydrate, which could lead to the post-translational modification of proteins (cytoplasmic, nuclear, and mitochondrial), It is affected by extracellular glucose concentration. O-GlcNAc glycosylation is the post-translational modification of proteins in response to high glucose levels. In the term of IPC, the levels of O-GlcNAc increased to produce cardioprotection. Jensen RV, et al., found that the levels of O-GlcNAc in isolated atrial trabeculae from non-diabetic volunteers' sample are augmented in the remote ischemic preconditioning group. However, diabetic persons failed to augment the already raised O-GlcNAc levels due to diabetes. It suggested that an increase in O-GlcNAc might confer cardioprotection. RIPC-induced cardioprotection depends on O-GlcNAc glycosylation and the levels of O-GlcNAc. However, among diabetic patients, O-GlcNAc glycosylation can't be increased again, even under RIPC treatment. Therefore, the effect of RIPC on cardioprotection is receded [70].
Diabetes would significantly abolish the cardioprotective effect of RIPC. In diabetic state, greater oxygen demand, mitochondrial uncoupling, reactive oxygen species (ROS) overproduction, and mitochondrial dysfunction, increases susceptibility to ischemia-reperfusion injury. The decrease in the release of humoral cardioprotective factors, O-GlcNAc signaling pathway, and also neural pathway damage might explain for attenuated cardioprotective effects of RIPC [68]. The possible mechanisms were summarized in Figure 3.
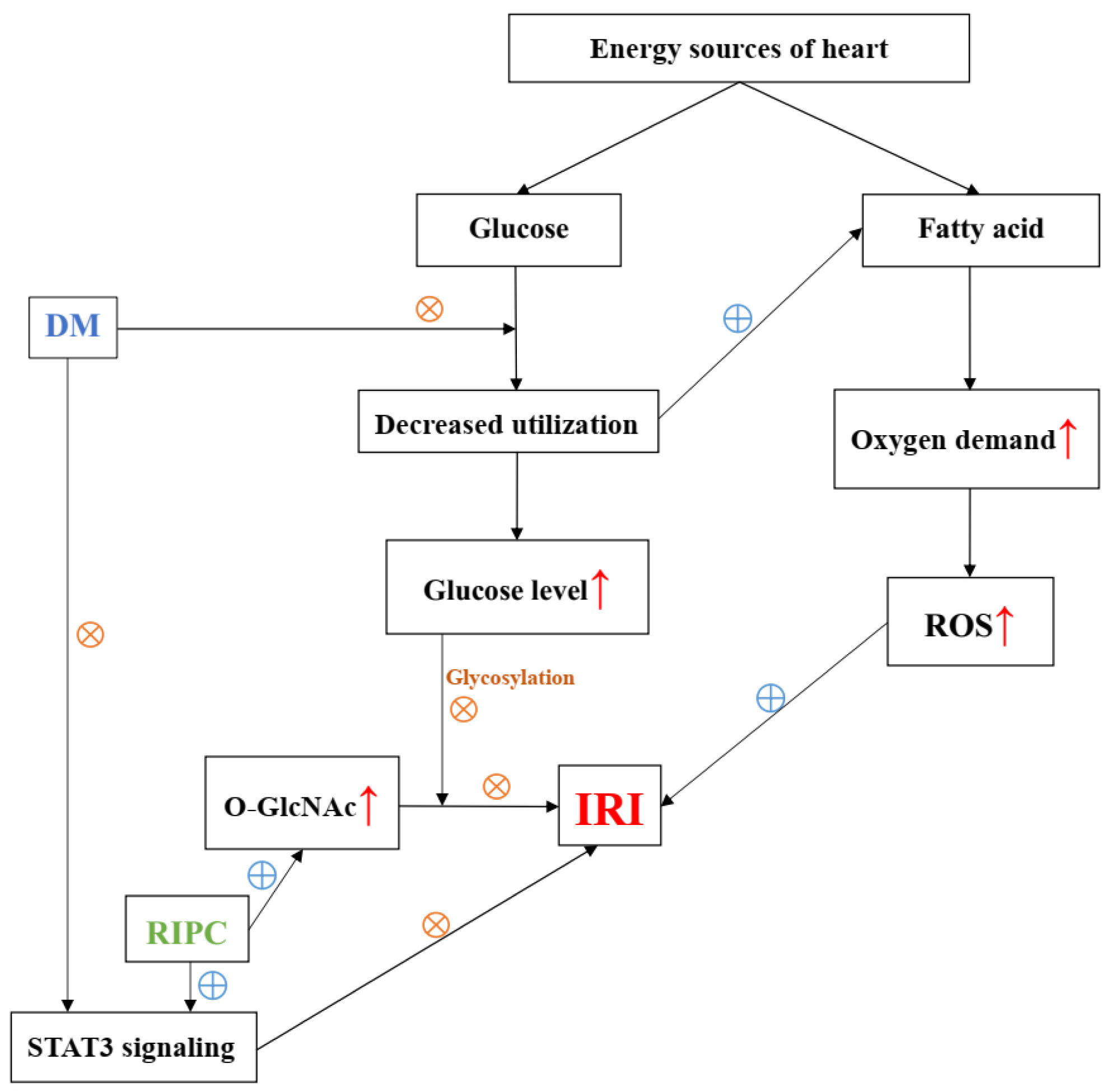
Figure 3: Diagram of possible mechanisms of diabetes mellitus that abolish the effect of RIPC.
Future Perspectives
RIPC has been shown in many researches to be a cardioprotective effect for attenuating IRI (ischemia/reperfusion injury) in animals or patients receiving reperfusion therapies. Its low cost and simplicity suggest its application in the future [6]. The benefit of RIPC on IRI remains controversial in clinical trials, though RIPC proves to be cardioprotective in many animal experiments [7]. New interest has been raised in confirming RIPC's benefit on IRI by detecting new mediator, such as extracellular vesicles [71]. Some clinical trials show that RIPC can reduce perioperative myocardial injury during elective PCI, CABG, acute ST-segment elevation myocardial infarction and other cardiac surgery procedures, [6,10,11,13,72,73] while recently some large multicentral researches produced negative results [7,9,48,74]. Some of them argued that the protocol of RIPC, anesthetic drugs and specific surgical procedures may account for the different outcomes of RIPC on clinical trials [11]. The current extensively used RIPC protocol (three or four cycles of 5 min ischemia and 5 min reperfusion in the left upper arm) and its application timing may not be the standardized setting. More large-scale clinical trials with long term follow-up study are required to determine whether RIPC benefits patients against IRI [7].
What's more, RIPC seems to elicit more systemic benefits than IPC (ischemic preconditioning), or IPostC (ischemic postconditioning), while most of studies focus on cardiac effect as their primary endpoints rather than other target-organ effect [13].
Also, most of the trials do not involve patients with comorbidities, such as diabetes, which is thought to be a high-risk group, as diabetes may interfere with cardioprotective signaling, making those patients more prone to myocardial ischemia/reperfusion [62]. But the interaction of hyperglycemia with RIPC or pharmacological or anesthetic pre/postconditioning needs further studies.
Many studies give concern to propofol, as its cardioprotective role in cells. Some trials thought that propofol may attenuate or abolish the cardioprotective effect of RIPC, but its specific mechanism and the relation with hyperglycemia has not yet to be determined [69].
In conclusion, identifying mechanism of current protective maneuvers or therapies for cardioprotection from IRI will still need further investigation, especially for diabetes. Also new cardioprotective targets and approaches to cardioprotection such as combination of multitarget therapy are to be discovered.
Summary
RIPC is a preferred clinical application to resist IRI. The possible cardioprotective mechanisms have been proposed but have not been fully proven. As its effect on clinical outcome is still controversial, further studies are needed to explore its clinical role.
References
- Reimer KA, Murry CE, Yamasawa I, Hill ML, Jennings RB. Four brief periods of myocardial ischemia cause no cumulative ATP loss or necrosis. Am J Physiol. 1986;251:H1306-15. PMID: 3789183
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 1986;74:1124-36. PMID: 3769170
- Kloner RA, Yellon D. Does ischemic preconditioning occur in patients? J. Am. Coll. Cardiol. 1994;24:1133-42. PMID: 7930208
- Kloner RA. Clinical application of remote ischemic preconditioning. Circulation 2009;119:776-8. PMID: 19221230
- Heusch G, Botker HE, Przyklenk K, Redington A, Yellon D. Remote ischemic conditioning. J. Am. Coll. Cardiol. 2015;65:177-95. PMID: 25593060
- Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sorensen HT, Redington AN, Nielsen TT. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 2010;375:727-34. PMID: 20189026
- Hausenloy DJ, Kharbanda R, Rahbek Schmidt M, Moller UK, Ravkilde J, Okkels Jensen L, Engstrom T, Garcia Ruiz JM, Radovanovic N, Christensen EF, Sorensen HT, Ramlall M, Bulluck H, Evans R, Nicholas J, Knight R, Clayton T, Yellon DM, Botker HE. Effect of remote ischaemic conditioning on clinical outcomes in patients presenting with an ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Eur. Heart J. 2015;36:1846-8. PMID: 26460398
- Hong DM, Mint JJ, Kim JH, Sohn IS, Lim TW, Lim YJ, Bahk JH, Jeon Y. The effect of remote ischaemic preconditioning on myocardial injury in patients undergoing off-pump coronary artery bypass graft surgery. Anaesth Intensive Care 2010;38:924-9. PMID: 20865880
- Li H, Irwin MG , Tao M, Li Y, Zhang L, Xia Z. Role of forkhead transcription factors in myocardial ischemic reperfusion injury in diabetes. J. Diabetes Metab. 2013:4. Doi: 10.4172/2155-6156.1000247
- Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, Price V, Tsagakis K, Neuhauser M, Peters J, Jakob H, Heusch G. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet 2013;382:597-604. PMID: 23953384
- Wang H, Lyu Y, Liao Q, Jin L, Xu L, Hu Y, Yu Y, Guo K. Effects of Remote Ischemic Preconditioning in Patients Undergoing Off-Pump Coronary Artery Bypass Graft Surgery. Front Physiol. 2019;10:495. PMID: 31110480
- Wu Q, Wang T, Chen S, Zhou Q, Li H, Hu N, Feng Y, Dong N, Yao S, Xia Z. Cardiac protective effects of remote ischaemic preconditioning in children undergoing tetralogy of fallot repair surgery: a randomized controlled trial. Eur. Heart J. 2018;39:1028-7. PMID: 28329231
- Deja MA, Piekarska M, Malinowski M, Wiaderkiewicz R, Czekaj P, Machej L, Weglarzy A, Kowalowka A, Kolodziej T, Czech E, Plewka D, Mizia M, Latusek T, Szurlej B. Can human myocardium be remotely preconditioned? The results of a randomized controlled trial. Eur J Cardiothorac Surg. 2019;55:1086-94. PMID: 30649238
- Kumar A, Singh H, Shariff M. Remote ischemic preconditioning and its role in the prevention of new onset atrial fibrillation post-cardiac surgery. A meta-analysis of randomized control trials. J Arrhythm. 2019;35:789-94. PMID: 31844467
- Figueira ERR, Rocha-Filho JA, Lanchotte C, Coelho AMM, Nakatani M, Tatebe ER, Lima JAV, Mendes CO, de Araujo B, Abdo EE, D'Albuquerque LC, Galvao FHF. Sevoflurane Preconditioning plus Postconditioning Decreases Inflammatory Response with Hemodynamic Recovery in Experimental Liver Ischemia Reperfusion. Gastroenterol Res Pract. 2019;2019:5758984. PMID: 31093276
- Xia Z, Huang Z, Ansley DM. Large-dose propofol during cardiopulmonary bypass decreases biochemical markers of myocardial injury in coronary surgery patients: a comparison with isoflurane. Anesth Analg. 2006;103:527-32. PMID: 16931656
- Huang Z, Zhong X, Irwin MG, Ji S, Wong GT, Liu Y, Xia ZY, Finegan BA, Xia Z. Synergy of isoflurane preconditioning and propofol postconditioning reduces myocardial reperfusion injury in patients. Clin Sci. 2011;121:57-69. PMID: 21291422
- Li T, Wu W, You Z, Zhou R, Li Q, Zhu D, Li H, Xiang X, Irwin MG, Xia Z, Liu J. Alternative use of isoflurane and propofol confers superior cardioprotection than using one of them alone in a dog model of cardiopulmonary bypass. Eur J Pharmacol. 2012;677:138-46. PMID: 22222823
- Merlocco AC, Redington KL, Disenhouse T, Strantzas SC, Gladstone R, Wei C, Tropak MB, Manlhiot C, Li J, Redington AN. Transcutaneous electrical nerve stimulation as a novel method of remote preconditioning: in vitro validation in an animal model and first human observations. Basic Res Cardiol. 2014;109:406. PMID: 24604614
- Jones WK, Fan GC, Liao S, Zhang JM, Wang Y, Weintraub NL, Kranias EG, Schultz JE, Lorenz J, Ren X. Peripheral nociception associated with surgical incision elicits remote nonischemic cardioprotection via neurogenic activation of protein kinase C signaling. Circulation 2009;120:S1-9. PMID: 19752352
- Wang C, Li H, Wang S, Mao X, Yan D, Wong SS, Xia Z, Irwin MG. Repeated Non-Invasive Limb Ischemic Preconditioning Confers Cardioprotection Through PKC-/STAT3 Signaling in Diabetic Rats. Cell Physiol Biochem. 2018;45:2107-21. PMID: 29533954
- Wong GT, Lu Y, Mei B, Xia Z, Irwin MG. Cardioprotection from remote preconditioning involves spinal opioid receptor activation. Life Sci. 2012;91:860-5. PMID: 22982345
- Lim SY, Yellon DM, Hausenloy DJ. The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol. 2010;105:651-5. PMID: 20449597
- Pickard JM, Davidson SM, Hausenloy DJ, Yellon DM. Co-dependence of the neural and humoral pathways in the mechanism of remote ischemic conditioning. Basic Res Cardiol. 2016;111:50. PMID: 27338249
- Lieder HR, Kleinbongard P, Skyschally A, Hagelschuer H, Chilian WM, Heusch G. Vago-Splenic Axis in Signal Transduction of Remote Ischemic Preconditioning in Pigs and Rats. Circulation research 2018;123:1152-63. PMID: 30359199
- Olenchock BA, Moslehi J, Baik AH, Davidson SM, Williams J, Gibson WJ, Chakraborty AA, Pierce KA, Miller CM, Hanse EA, Kelekar A, Sullivan LB, Wagers AJ, Clish CB, Vander Heiden MG, Kaelin WG, Jr. EGLN1 Inhibition and Rerouting of alpha-Ketoglutarate Suffice for Remote Ischemic Protection. Cell 2016;164:884-95. PMID: 26919427
- Weinbrenner C, Schulze F, Sarvary L, Strasser RH. Remote preconditioning by infrarenal aortic occlusion is operative via delta1-opioid receptors and free radicals in vivo in the rat heart. Cardiovasc Res. 2004;61:591-9. PMID: 14962489
- Giricz Z, Varga ZV, Baranyai T, Sipos P, Paloczi K, Kittel A, Buzas EI, Ferdinandy P. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J Mol Cell Cardiol. 2014;68:75-8. PMID: 24440457
- Kleinbongard P, Skyschally A, Heusch G. Cardioprotection by remote ischemic conditioning and its signal transduction. Pflugers Arch. 2017;469:159-81. PMID: 27928644
- Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G, Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res. 2014;114:1601-10. PMID: 24643960
- Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res. 2015;116:674-99. PMID: 25677517
- Cai ZP, Parajuli N, Zheng X, Becker L. Remote ischemic preconditioning confers late protection against myocardial ischemia-reperfusion injury in mice by upregulating interleukin-10. Basic Res Cardiol. 2012;107:277. PMID: 22752341
- Davidson SM, Selvaraj P, He D, Boi-Doku C, Yellon RL, Vicencio JM, Yellon DM. Remote ischaemic preconditioning involves signalling through the SDF-1alpha/CXCR4 signalling axis. Basic Res Cardiol. 2013;108:377. PMID: 23917520
- Minghua W, Zhijian G, Chahua H, Qiang L, Minxuan X, Luqiao W, Weifang Z, Peng L, Biming Z, Lingling Y, Zhenzhen W, Jianqing X, Huihui B, Xiaozhong W, Xiaoshu C. Plasma exosomes induced by remote ischaemic preconditioning attenuate myocardial ischaemia/reperfusion injury by transferring miR-24. Cell Death Dis. 2018;9:320. PMID: 29476052
- Kalakech H, Hibert P, Prunier-Mirebeau D, Tamareille S, Letournel F, Macchi L, Pinet F, Furber A, Prunier F. RISK and SAFE signaling pathway involvement in apolipoprotein A-I-induced cardioprotection. PloS One 2014;9:e107950. PMID: 25237809
- Hausenloy DJ, Iliodromitis EK, Andreadou I, Papalois A, Gritsopoulos G, Anastasiou-Nana M, Kremastinos DT, Yellon DM. Investigating the signal transduction pathways underlying remote ischemic conditioning in the porcine heart. Cardiovasc Drugs Ther. 2012;26:87-93. PMID: 22207395
- Chen H, Jing XY, Shen YJ, Wang TL, Ou C, Lu SF, Cai Y, Li Q, Chen X, Ding YJ, Yu XC, Zhu BM. Stat5-dependent cardioprotection in late remote ischaemia preconditioning. Cardiovasc Res. 2018;114:679-89. PMID: 29365089
- McLeod SL, Iansavichene A, Cheskes S. Remote Ischemic Perconditioning to Reduce Reperfusion Injury During Acute ST-Segment-Elevation Myocardial Infarction: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2017;6:e005522. PMID: 28515120
- Pei H, Wu Y, Wei Y, Yang Y, Teng S, Zhang H. Remote ischemic preconditioning reduces perioperative cardiac and renal events in patients undergoing elective coronary intervention: a meta-analysis of 11 randomized trials. PloS One 2014;9:e115500. PMID: 25551671
- Zografos TA, Katritsis GD, Katritsis DG. Remote ischemic preconditioning reduces peri-procedural myocardial injury in elective percutaneous coronary intervention: a meta-analysis. Int J Cardiol. 2014;173:530-2. PMID: 24681008
- Davies WR, Brown AJ, Watson W, McCormick LM, West NE, Dutka DP, Hoole SP. Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: the CRISP stent trial long-term follow-up. Circ Cardiovasc Interv. 2013;6:246-51. PMID: 23696599
- Prasad A, Gossl M, Hoyt J, Lennon RJ, Polk L, Simari R, Holmes DR, Jr., Rihal CS, Lerman A. Remote ischemic preconditioning immediately before percutaneous coronary intervention does not impact myocardial necrosis, inflammatory response, and circulating endothelial progenitor cell counts: a single center randomized sham controlled trial. Catheter Cardiovasc Interv. 2013;81:930-6. PMID: 22517646
- Pryds K, Bottcher M, Sloth AD, Munk K, Rahbek Schmidt M, Botker HE, Investigators C. Influence of preinfarction angina and coronary collateral blood flow on the efficacy of remote ischaemic conditioning in patients with ST segment elevation myocardial infarction: post hoc subgroup analysis of a randomised controlled trial. BMJ Open 2016;6:e013314. PMID: 27884851
- Candilio L, Malik A, Ariti C, Barnard M, Di Salvo C, Lawrence D, Hayward M, Yap J, Roberts N, Sheikh A, Kolvekar S, Hausenloy DJ, Yellon DM. Effect of remote ischaemic preconditioning on clinical outcomes in patients undergoing cardiac bypass surgery: a randomised controlled clinical trial. Heart 2015;101:185-92. PMID: 25252696
- Wu Q, Wang T, Chen S, Zhou Q, Li H, Hu N, Feng Y, Dong N, Yao S, Xia Z. Cardiac protective effects of remote ischaemic preconditioning in children undergoing tetralogy of fallot repair surgery: a randomized controlled trial. Eur Heart J. 2018;39:1028-37. PMID: 28329231
- Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, Ashley E, Vichare S, Di Salvo C, Kolvekar S, Hayward M, Keogh B, MacAllister RJ, Yellon DM. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet 2007;370:575-9. PMID: 17707752
- Benstoem C, Stoppe C, Liakopoulos OJ, Ney J, Hasenclever D, Meybohm P, Goetzenich A. Remote ischaemic preconditioning for coronary artery bypass grafting (with or without valve surgery). Cochrane Database Syst Rev. 2017;5:CD011719. PMID: 28475274
- Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM, Investigators ET. Remote Ischemic Preconditioning and Outcomes of Cardiac Surgery. N Engl J Med. 2015;373:1408-17. PMID: 26436207
- Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K, Redington AN, MacAllister R. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 2002;106:2881-3. PMID: 12460865
- Gunaydin B, Cakici I, Soncul H, Kalaycioglu S, Cevik C, Sancak B, Kanzik I, Karadenizli Y. Does remote organ ischaemia trigger cardiac preconditioning during coronary artery surgery? Pharmacol Res. 2000;41:493-6. PMID: 10704275
- Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, Holtby HM, Cox PN, Smallhorn JF, Van Arsdell GS, Redington AN. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006;47:2277-82. PMID: 16750696
- Domanski MJ, Mahaffey K, Hasselblad V, Brener SJ, Smith PK, Hillis G, Engoren M, Alexander JH, Levy JH, Chaitman BR, Broderick S, Mack MJ, Pieper KS, Farkouh ME. Association of myocardial enzyme elevation and survival following coronary artery bypass graft surgery. JAMA 2011;305:585-91. PMID: 21304084
- Remote Preconditioning Trialists G, Healy DA, Khan WA, Wong CS, Moloney MC, Grace PA, Coffey JC, Dunne C, Walsh SR, Sadat U, Gaunt ME, Chen S, Tehrani S, Hausenloy DJ, Yellon DM, Kramer RS, Zimmerman RF, Lomivorotov VV, Shmyrev VA, Ponomarev DN, Rahman IA, Mascaro JG, Bonser RS, Jeon Y, Hong DM, Wagner R, Thielmann M, Heusch G, Zacharowski K, Meybohm P, Bein B, Tang TY. Remote preconditioning and major clinical complications following adult cardiovascular surgery: systematic review and meta-analysis. Int J Cardiol. 2014;176:20-31. PMID: 25022819
- Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev. 2014;66:1142-74. PMID: 25261534
- Kocsis GF, Pipis J, Fekete V, Kovacs-Simon A, Odendaal L, Molnar E, Giricz Z, Janaky T, van Rooyen J, Csont T, Ferdinandy P. Lovastatin interferes with the infarct size-limiting effect of ischemic preconditioning and postconditioning in rat hearts. Am J Physiol Heart Circ Physiol. 2008;294:H2406-9. PMID: 18359895
- Pierce B, Bole I, Patel V, Brown DL. Clinical Outcomes of Remote Ischemic Preconditioning Prior to Cardiac Surgery: A Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc. 2017;6: e004666. PMID: 28219918
- Gaspar A, Lourenco AP, Pereira MA, Azevedo P, Roncon-Albuquerque R, Jr., Marques J, Leite-Moreira AF. Randomized controlled trial of remote ischaemic conditioning in ST-elevation myocardial infarction as adjuvant to primary angioplasty (RIC-STEMI). Basic Res Cardiol. 2018;113:14. PMID: 29516192
- Wang X, Kong N, Zhou C, Mungun D, Iyan Z, Guo Y, Yang Z. Effect of Remote Ischemic Preconditioning on Perioperative Cardiac Events in Patients Undergoing Elective Percutaneous Coronary Intervention: A Meta-Analysis of 16 Randomized Trials. Cardiol Res Pract. 2017;2017:6907167. PMID: 29062582
- Ali ZA, Callaghan CJ, Lim E, Ali AA, Nouraei SA, Akthar AM, Boyle JR, Varty K, Kharbanda RK, Dutka DP, Gaunt ME. Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation 2007;116:I98-105. PMID: 17846333
- Walsh SR, Sadat U, Boyle JR, Tang TY, Lapsley M, Norden AG, Gaunt ME. Remote ischemic preconditioning for renal protection during elective open infrarenal abdominal aortic aneurysm repair: randomized controlled trial. Vasc Endovascular Surg. 2010;44:334-40. PMID: 20484066
- Li C, Li YS, Xu M, Wen SH, Yao X, Wu Y, Huang CY, Huang WQ, Liu KX. Limb remote ischemic preconditioning for intestinal and pulmonary protection during elective open infrarenal abdominal aortic aneurysm repair: a randomized controlled trial. Anesthesiology 2013;118:842-52. PMID: 23353795
- Mao X, Ge Z, Xie X, Lian Q, Xia Z. Propofol Cardioprotection against Myocardial Ischemia-Reperfusion Injury: A Mechanistic Review. React. Oxygen Species 2016;1:178-188. DOI: 10.20455/ros.2016.831
- Kim DH, Kim YJ, Chang SA, Lee HW, Kim HN, Kim HK, Chang HJ, Sohn DW, Park YB. The protective effect of thalidomide on left ventricular function in a rat model of diabetic cardiomyopathy. Eur J Heart Fail. 2010;12:1051-60. PMID: 20601373
- Leung AA, Eurich DT, Lamb DA, Majumdar SR, Johnson JA, Blackburn DF, McAlister FA. Risk of heart failure in patients with recent-onset type 2 diabetes: population-based cohort study. J Card Fail. 2009;15:152-7. PMID: 19254675
- Wang Y, Li H, Huang H, Liu S, Mao X, Wang S, Wong SS, Xia Z, Irwin MG. Cardioprotection from emulsified isoflurane postconditioning is lost in rats with streptozotocin-induced diabetes due to the impairment of Brg1/Nrf2/STAT3 signalling. Clin Sci. 2016;130:801-12. PMID: 26846682
- Deng F, Wang S, Zhang L, Xie X, Cai S, Li H, Xie GL, Miao HL, Yang C, Liu X, Xia Z. Propofol Through Upregulating Caveolin-3 Attenuates Post-Hypoxic Mitochondrial Damage and Cell Death in H9C2 Cardiomyocytes During Hyperglycemia. Cell Physiol Biochem. 2017;44:279-92. PMID: 29130958
- Zhu XH, Yuan HJ, Wu YN, Kang Y, Jiao JJ, Gao WZ, Liu YX, Lou JS, Xia Z. Non-invasive limb ischemic pre-conditioning reduces oxidative stress and attenuates myocardium ischemia-reperfusion injury in diabetic rats. Free Radic Res. 2011;45:201-10. PMID: 20942563
- Tyagi S, Singh N, Virdi JK, Jaggi AS. Diabetes abolish cardioprotective effects of remote ischemic conditioning: evidences and possible mechanisms. J Physiol Biochem. 2019;75:19-28. PMID: 30729392
- Jin HY, Baek HS, Park TS. Morphologic Changes in Autonomic Nerves in Diabetic Autonomic Neuropathy. Diabetes Metab J. 2015;39:461-7. PMID: 26706915
- Jensen RV, Zachara NE, Nielsen PH, Kimose HH, Kristiansen SB, Botker HE. Impact of O-GlcNAc on cardioprotection by remote ischaemic preconditioning in non-diabetic and diabetic patients. Cardiovasc Res. 2013;97:369-78. PMID: 23201773
- Frey UH, Klaassen M, Ochsenfarth C, Murke F, Thielmann M, Kottenberg E, Kleinbongard P, Klenke S, Engler A, Heusch G, Giebel B, Peters J. Remote ischaemic preconditioning increases serum extracellular vesicle concentrations with altered micro-RNA signature in CABG patients. Acta Anaesthesiol Scand. 2019;63:483-92. PMID: 30548252
- Eitel I, Stiermaier T, Rommel KP, Fuernau G, Sandri M, Mangner N, Linke A, Erbs S, Lurz P, Boudriot E, Mende M, Desch S, Schuler G, Thiele H. Cardioprotection by combined intrahospital remote ischaemic perconditioning and postconditioning in ST-elevation myocardial infarction: the randomized LIPSIA CONDITIONING trial. Eur Heart J. 2015;36:3049-57. PMID: 26385956
- Traverse JH, Swingen CM, Henry TD, Fox J, Wang YL, Chavez IJ, Lips DL, Lesser JR, Pedersen WR, Burke NM, Pai A, Lindberg JL, Garberich RF. NHLBI-Sponsored Randomized Trial of Postconditioning During Primary Percutaneous Coronary Intervention for ST-Elevation Myocardial Infarction. Circ Res. 2019;124:769-78. PMID: 30602360
- Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, Coburn M, Schaelte G, Boning A, Niemann B, Roesner J, Kletzin F, Strouhal U, Reyher C, Laufenberg-Feldmann R, Ferner M, Brandes IF, Bauer M, Stehr SN, Kortgen A, Wittmann M, Baumgarten G, Meyer-Treschan T, Kienbaum P, Heringlake M, Schon J, Sander M, Treskatsch S, Smul T, Wolwender E, Schilling T, Fuernau G, Hasenclever D, Zacharowski K, Collaborators RIS. A Multicenter Trial of Remote Ischemic Preconditioning for Heart Surgery. N Engl J Med. 2015;373:1397-407. PMID: 26436208
Table of Contents
- Abstract
- Myocardial Ischemia and Reperfusion Injury
- Definition and differences of Conditionings IPC, RIPC and APC that are Applicable During Cardiac Surgery
- Potential Mechanism of RIPC
- Debate on clinical trials of RIPC (negative/positive)
- Modes of RIPC and its Evolution
- Impacts of Diabetes on the Effectiveness of RIPC in Cardiac Surgery
- Future Perspectives
- Summary
- Figure 1
- Figure 2
- Figure 3
- Table 1
- References