Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/144
Case Report | Volume 8 | Issue 4 Open Access
Anesthetic Management of Robotic Adrenalectomy for Pheochromocytoma: A Case Report and Discussion
Joshua Spiro, MD* and Gurmukh Sahota, MD, PhD
Department of Anesthesiology and Critical Care, Hospital of the University of Pennsylvania, Philadelphia, PA 19104, USA
Joshua Spiro, MD, Department of Anesthesiology and Critical Care, Hospital of the University of Pennsylvania, 3400 Spruce Street, 6th Floor Dulles Building, Philadelphia, PA 19104, USA, Tel: 1(215)-327-6793, E-mail: joshaspiro@gmail.comEditor: Li-ming Zhang MD, Associate Professor, 200 Lothrop Street, UPMC Montefiore, Anesthesiology, Suite 467.2, Pittsburgh, PA 15213, USA, E-mail: zhangl1@anes.upmc.edu
Received: May 22, 2021 | Accepted: September 02, 2021 | Published: October 12, 2021
Citation: Spiro J, Sahota G. Anesthetic Management of Robotic Adrenalectomy for Pheochromocytoma: A Case Report and Discussion. Transl Perioper & Pain Med 2021; 8(4):375-380
Abstract
Pheochromocytomas may secrete a multitude of vasoactive, chronotropic, and inotropic mediators such as norepinephrine and epinephrine. Consequently, these substrates may cause significant hemodynamic variation in the perioperative setting requiring careful preoperative, intraoperative, and postoperative care. In this report, we examine the case of a female patient in her 60s presenting for robotic adrenalectomy for pheochromocytoma. We then review the different stages of management of the patient throughout the perioperative period and the anesthetic and surgical considerations in managing each stage.
Keywords
Adrenalectomy, Pheochromocytoma, Robotic-assisted
Introduction
Pheochromocytomas are catecholamine-secreting neoplasms originating from the adrenal medulla with an incidence of roughly 2-8 cases per million [1]. The catecholamines released by these tumors can induce abrupt surges in heart rate and blood pressure which may result in hypertensive crisis. Due to surgical advancements, robotic-assisted adrenalectomy is commonly performed to remove pheochromocytoma. While the surgical stimulation is much less compared to the traditional open adrenalectomy, significant hemodynamic disturbance can still be seen in the perioperative period. This case highlights a female patient in her 60s presenting for robotic adrenalectomy for pheochromocytoma and the accompanying hemodynamic fluctuations associated with the condition. We will also discuss some specific anesthesia considerations for robotic-assisted adrenalectomy. Since there is no patient identifiable information provided in this case, no IRB approval was required.
Case Description
An ASA 2 female patient in her 60s with an incidentally found pheochromocytoma presented for a robotic adrenalectomy. Her weight was 78.5 kg with a BMI of 29.7. The patient initially presented to her primary care physician with right-sided abdominal pain, for which an ultrasound and subsequent MRI were performed demonstrating a 4.4 cm right-sided adrenal mass. She demonstrated greater than four metabolic equivalents and denied symptoms associated with increased catecholamines including light headedness, dizziness, vomiting, palpitations, diaphoresis, or flushing. Laboratory analysis found that she had elevated plasma normetanephrine levels at 1903.8 (normal 0-191.8 pg/mL) and normal metanephrine levels at 52.1 (normal range 0-88 pg/mL) leading to the diagnosis of a pheochromocytoma. Past medical history was significant only for hypertension which was treated with lisinopril 10 mg daily. After consultation with urology, our patient decided to proceed with right robotic adrenalectomy. Preoperatively, she was placed on phenoxybenzamine for a total of 17 days prior to surgery for a blood pressure goal of 120/80. After appropriate blood pressure control, she was subsequently placed on metoprolol for a heart rate goal of 60-80 bpm.
On presentation to the operating room suite, routine non-invasive monitoring was established, including non-invasive blood pressure (NBP), heart rate (HR), pulse oximetry (SpO2), and electrocardiography (ECG). Baseline blood pressure was 165/81 mmHg, pulse oximetry was 99%, heart rate was 74 beat/min, and ECG demonstrated normal sinus rhythm. The patient was given 2 mg of midazolam for anxiolysis and a left radial arterial line was placed. The patient was induced with 200 mcg of fentanyl, 80 mg of lidocaine, 240 mg total of propofol, and 80 mg of rocuronium. She was maintained on sevoflurane for the duration of the procedure. Bilateral upper extremity 16g IVs were placed for large volume access and a triple lumen catheter in the right internal jugular vein was placed for postoperative vasopressor administration.
The patient was given 1 milligram of hydromorphone prior to incision and docking of the da Vinci Xi robotic surgical platform (Intuitive surgical, Sunnyvale, CA, USA). The patient was relatively hemodynamically stable without dramatic fluctuation throughout the induction, positioning, and before adrenal gland manipulation. On dissection down to the adrenal gland, the blood pressure gradually increased to a peak of 190/86 just prior to clamping of the effluent vein (Figure 1). The patient was given the total boluses of 1.2 mg nicardipine, 20 mg of esmolol, 60 mg of propofol during this period and placed on a 5 mg/hour nicardipine infusion. After clamping of the effluent vein, the blood pressure then continued in the range of 106-140 mmHg systolic over 69-97 mmHg diastolic. On removal of the pheochromocytoma specimen, the blood pressure dropped quickly to 91/56, requiring vasopressin and phenylephrine boluses followed by phenylephrine infusion throughout the remainder of the case. At the conclusion of the surgery, neuromuscular blockade was reversed, and the patient was extubated. She was then taken to the surgical intensive care unit for further monitoring. She was weaned off phenylephrine infusion shortly after transport to SICU where she stayed overnight. After transfer to the floor the following day, the patient was discharged from the hospital on postoperative day 2.
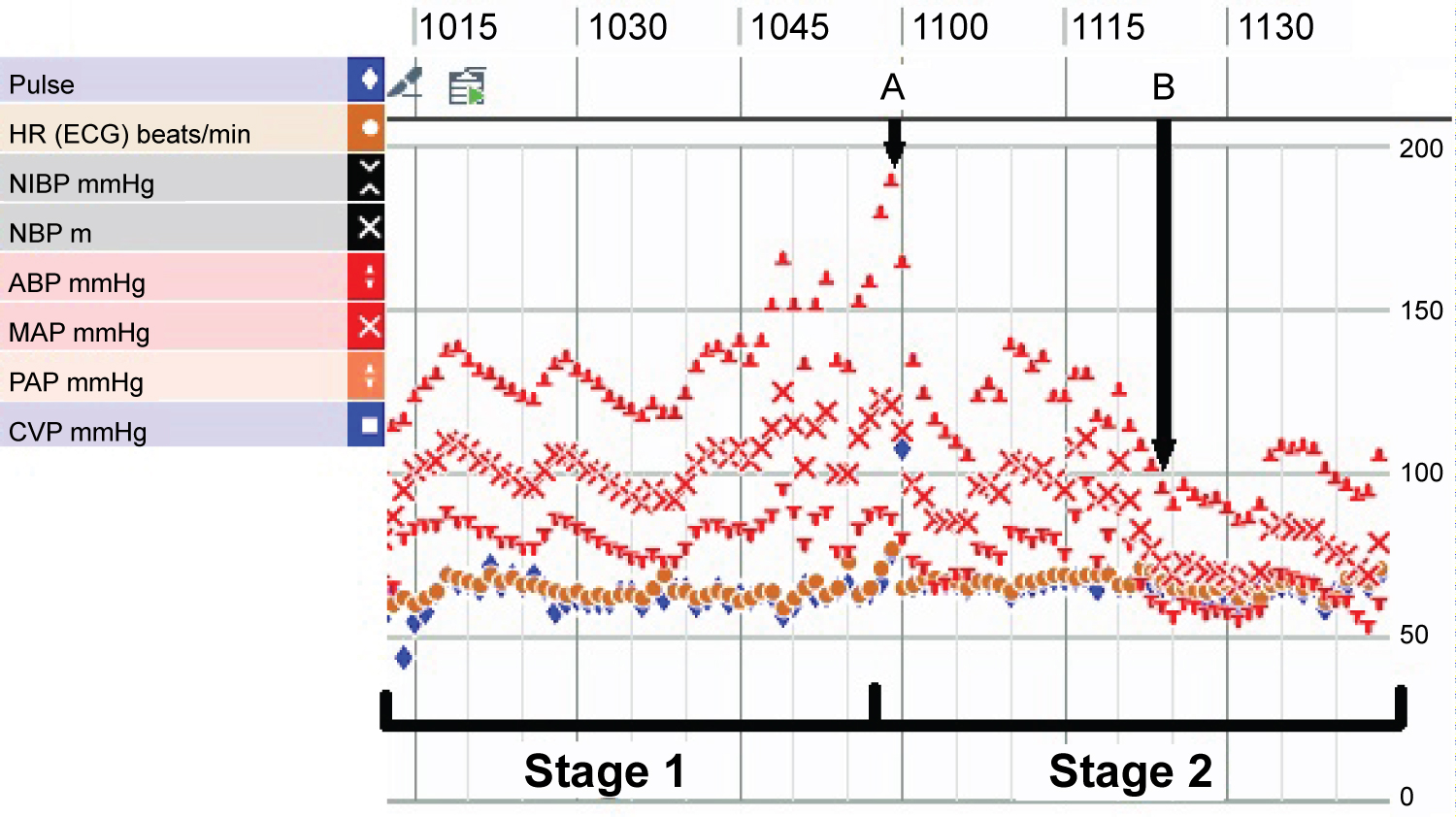
Figure 1: Intraoperative vital signs and hemodynamic variability during stage one and two of surgery. "A" represents clamping of the effluent vein and "B" represents removal of entire pheochromocytoma specimen.
Discussion and Review
Preoperative considerations
The perioperative management of the pheochromocytoma starts with preoperative optimization. Plasma metanephrine and normetanephrine levels (the metabolites of epinephrine and norepinephrine, respectively) are ascertained in consultation with an endocrinologist; the treatment ramifications of these levels are further discussed in the Pharmacologic Agents section. Even when patients present asymptomatically, they should have the same preoperative laboratory testing as symptomatic patients as they manifest similar levels of hemodynamic instability to symptomatic patients [2]. Once the pheochromocytoma is diagnosed, alpha-adrenergic blockade (typically with phenoxybenzamine) is administered starting 10-14 days prior to the operation to normalize blood pressure. Intravascular volume is carefully repleted after successful alpha blockade. Most hospitals utilize some variation on the 1982 Roizen criteria [3] to determine adequacy of alpha blockade which include:
1. Blood pressure should not be > 160/90 mmHg for 24 hours prior to surgery.
2. Orthostatic hypotension should be present, but blood pressure should not be lower than < 80/45 mmHg.
3. EKG should be free of ST or T wave changes for a week a week prior to surgery.
4. No more than one PVC should occur every five minutes.
The heart rate may then be normalized with beta blockade subsequently; however, never prior to alpha blockade as this may result in unopposed alpha receptor stimulation causing hypertensive crisis. Preoperative cardiac evaluation should then be performed with electrocardiogram as well as echocardiogram for symptomatic patients. Some authors advocate for preoperative echocardiography for every patient with pheochromocytoma resection to identify dilated cardiomyopathy, diastolic dysfunction, or catecholamine induced cardiomyopathy (Takotsubo's) [4]. Given our asymptomatic patient with a very active lifestyle, we elected to not perform echocardiography prior to performing the patient's anesthetic in accordance with the AHA guidelines for perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery [5].
Surgical approach and stages
Adrenalectomy may be performed via either a minimally invasive or open approach with important implications for anesthetic management. The minimally invasive surgical approach includes laparoscopic or robotic-assisted resection which is the most suitable approach for adrenal masses < 15 cm in diameter and is associated with less hemodynamic instability, less catecholamine release, less pain, and shorter hospital stay than an open approach (see Table 1) [6,7]. Within the minimally invasive approach, the surgeon may elect to perform the procedure from either a transabdominal or a retroperitoneal approach. Of the minimally invasive techniques, the laparoscopic approach is the current gold standard; however, increasing availability and familiarity with the robotic platform has led to more widespread use of the robotic-assisted technique [7]. The robotic-assisted approach allows for improved 3D visualization, tremor filtration, and trocar "wrist" joint leading to improved dexterity for the surgical team. Early studies show that the robotic approach may have better outcomes than the laparoscopic approach as shown in Table 2 [8-10]. Despite these outcomes, Ma, et al. demonstrated an increased total hospitalization cost of $8869.90 for robotic-assisted vs. $4721.80 for laparoscopic adrenalectomy for pheochromocytoma [10]. The anesthetic considerations for the robotic-assisted approach are similar to that of the laparoscopic approach. Once the da Vinci Xi robot is docked, the anesthesiologist will have difficulty reaching the patients' tucked arms due to the robot's physical presence. Therefore, proper access should be obtained prior to positioning. Decreased surgical time and lower pain scores associated with the robotic approach may require less narcotic medication for a safer and more efficient emergence from anesthesia. Pain control may be treated with a multimodal approach utilizing opioids, NSAIDs, and acetaminophen. Regional techniques such as spinal, epidural, or truncal nerve blocks, such as transverse abdominus plane, are rarely required for pain control unless conversion to an open procedure is performed.
Table 1: Differences in clinical parameters between minimally invasive and open surgical approaches to pheochromocytoma.
| Parameters | MIS | Open |
|---|---|---|
| ICU Stay (days) | Lower | Higher |
| Hospital Stay (days) | Lower | Higher |
| Tumor Size | Smaller | Larger |
| Catecholamine Release | Low | Higher |
| Intraoperative IV fluid | Lower | Higher |
| Operative Time | Shorter | Longer |
| EBL | Lower | Higher |
| OR end mean BP (mmHg) | Higher | Lower |
| Pain | Lower | Higher |
Table 2: Differences in clinical parameters between laparoscopic and robotic-assisted approaches to pheochromocytoma.
| Parameters | Laparoscopic | Robotic-assisted |
|---|---|---|
| ICU Stay | Longer | Shorter |
| Operative Time | Longer | Shorter |
| Pain Score | Higher | Lower |
| Total Hospitalization Cost | Lower | Higher |
Laparoscopic or robotic-assisted adrenalectomy involves two intraoperative stages, demarcated by the ligation of the effluent vein. The first stage is characterized as the portion prior to the ligation of the effluent vein including intubation, insufflation, and tumor manipulation. As the effluent vein has yet to be ligated, this phase is characterized by potentially significant catecholamine release leading to hypertension, tachycardia, and arrhythmias that can necessitate vasodilating and antiarrhythmic medications. If elevations in blood pressure are inadequately treated, this can result in a hypertensive crisis. The second stage is the portion after ligation of the effluent vein. The sudden withdrawal of the catecholamine stimulus commonly results in rebound hypotension necessitating vasopressor medications and fluid bolus. However, during our case, the blood pressure didn't fully nadir until the pheochromocytoma specimen was fully removed (as demonstrated by B in Figure 1).
Monitoring and access
Prior to the procedure, premedication with a benzodiazepine may be considered to preclude sympathetic surges from anxiety. Standard monitors should be placed, and pre-induction arterial cannulation should be performed for beat-to-beat blood pressure monitoring and frequent lab draws [4]. Though arterial line is typically placed prior to induction to monitor hemodynamics during intubation, studies have not shown a significant difference in catecholamine release before and immediately after intubation [11]. Central venous catheter may be placed for administration of vasoactive agents both intraoperatively and later in the intensive care unit. However, there is no consensus as to which patients warrant central line placement. One study of two large academic institutions demonstrated central lines were placed in 30% of patients at one institution and 59.5% of patients in the other institution [12]. During our case, we placed a triple lumen catheter after discussion with the urology team regarding the possibility of postoperative pressor requirement as well as bilateral 16-gauge for rapid fluid resuscitation. Pulmonary artery catheter and transesophageal echocardiogram may also be considered if patients have additional comorbidities such as catecholamine induced cardiomyopathy and right ventricular failure [13].
Pharmacologic agents
The procedure is most often performed under general anesthesia utilizing a variety of agents such as propofol, etomidate, opioids, neuromuscular blockers, and alpha blockers such as dexmedetomidine. However, agents such as ketamine, and morphine are typically avoided due to increased sympathetic stimulation and histamine release, respectively [14]. Regional anesthesia (epidural) has been performed for open pheochromocytoma resections; however, is not required for minimally invasive adrenalectomy. While anesthetic and analgesic medications may preclude large hemodynamic swings from stimulation, they do not necessarily prevent hemodynamic changes from tumor manipulation. TIVA with propofol infusion has been used safely during adrenalectomy [15]; however, no study exists comparing the hemodynamic stability of TIVA and volatile anesthetic maintenance in these cases.
The primary catecholamine secreted by the pheochromocytoma has important implications for the anesthesiologists' management. If the tumor primarily secretes epinephrine, then one should be prepared to treat both hypertension and tachycardia. However, if the tumor primarily secretes norepinephrine, then hypertension is more common than tachycardia due to the increased ratio of alpha agonism to beta agonism. To prevent and treat hypertension, an array of medications need to be readily available, however, short-acting medications should be considered as the first line of choices. Calcium channel blockers (nicardipine or clevidipine), nitroprusside, and phentolamine may be utilized for vasodilation. Phentolamine, a reversible alpha antagonist, should be used judiciously as the alpha blockade may worsen hypotension after tumor removal. In our case, we used nicardipine for both bolus and infusion as our primary antihypertensive agent. In case of hypertensive crisis, rapid bolus of vasodilating agents must be administered to avoid complications such as pulmonary edema, heart failure, acute kidney injury, etc. Throughout the procedure, the anesthesiologist should be in open communication with the surgical team to predict, preempt, and treat hemodynamically significant events as shown in Figure 2.
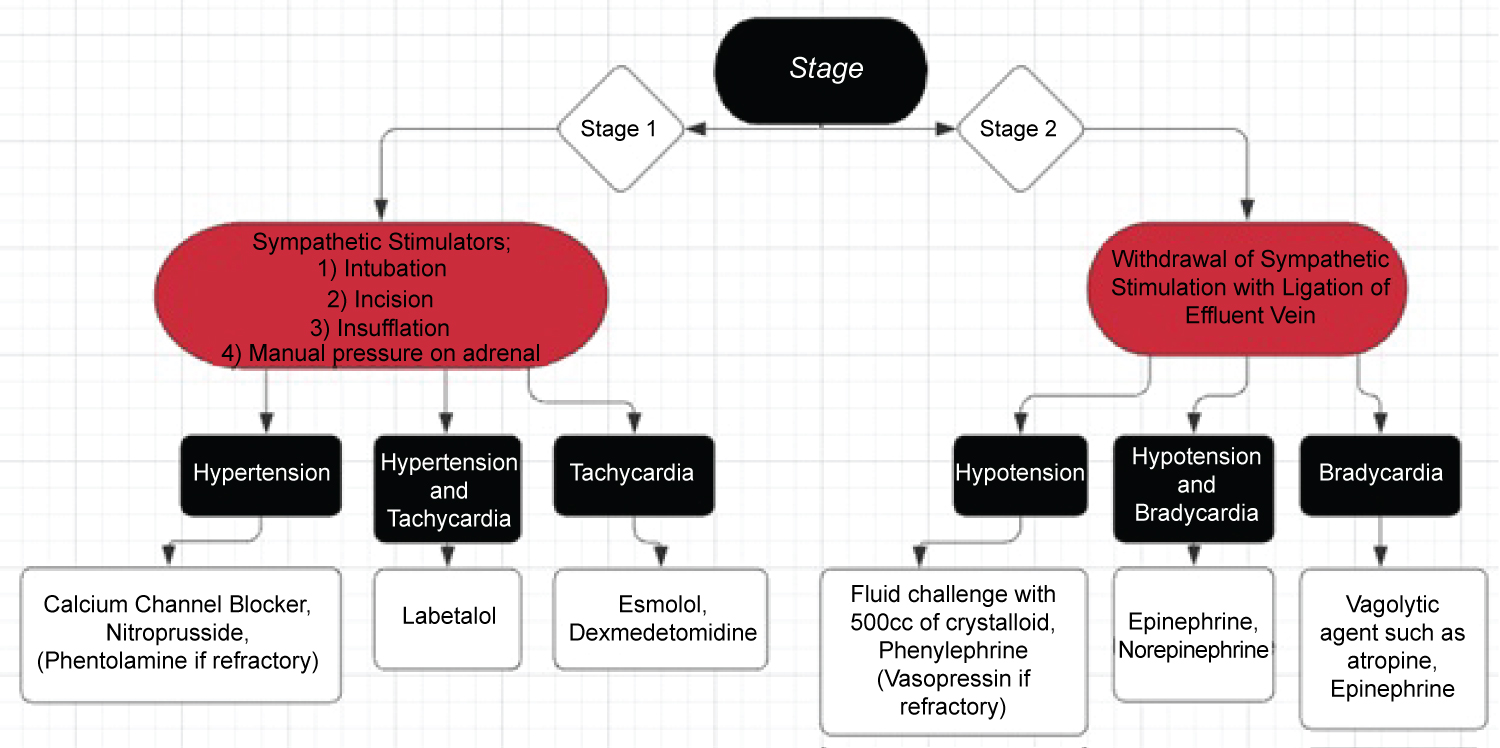
Figure 2: Treatment schema for hemodynamically significant events during each stage of the procedure.
Esmolol, labetalol, lidocaine, and amiodarone may be used to treat both tachycardia and arrhythmias. Magnesium is a unique agent which can prevent secretion of catecholamines from the adrenal medulla and antagonize alpha-adrenergic receptors. However, it is unclear whether patients that have already had preoperative alpha blockade benefit significantly from intraoperative magnesium use [16]. Dexmedetomidine, an α2-adrenergic agonist, has also been used successfully during pheochromocytoma resection in combination with other antihypertensive agents [17]; however, no study to date has determined whether it is more effective than other anesthetic agents in patients already treated preoperatively with alpha blockade.
During the second stage of the surgery, the ligation of the effluent vein results in a sudden drop in catecholamine release resulting in significant and sometime refractory hypotension. To support blood pressure, vasopressor agents such as phenylephrine, norepinephrine, and/or vasopressin should be readily available as both bolus and infusion for significant and refractory hypotension. Depending on the patient's preoperative cardiac function and baseline heart rate, epinephrine may also be utilized to improve contractility and vascular tone. Previous evidence indicated that patients would benefit from liberal fluid administration after pheochromocytoma resection due to volume depletion from chronic alpha agonism [18]. However, more recent evidence utilizing esophageal doppler monitoring found that patients tend to be vasoplegic rather than hypovolemic after resection [19].
Postoperative management
Postoperatively, patients may have hypertension due to pain or retained tumor tissue, or hypotension due to vasoplegia, hypovolemia, arrhythmia, or decreased inotropy. Patients with hemodynamic instability that require continued intervention must be transported to the intensive care unit for close hemodynamic observation. Thompson, et al. found that patients that were placed on preoperative beta-blockade therapy was an independent risk for postoperative hemodynamic instability and patients who undergo laparoscopic adrenalectomy for pheochromocytoma < 5 cm are unlikely to need postoperative vasopressor therapy [20]. Other complications in the postoperative period may include adrenocortical insufficiency, acute kidney injury, hypoglycemia, and congestive heart failure [21]. Given these factors, it is commonly recommended that patients be admitted to an intensive care unit in the postoperative setting. However, it is left to the discretion of the multidisciplinary team whether patients that had an uneventful intraoperative course (otherwise healthy patients with minimal blood loss, and minimal hemodynamic instability intraoperatively) warrant admission to the intensive care unit after surgery. Though our patient was placed on preoperative beta blockade, we were able to wean her phenylephrine infusion off within fifteen minutes of reaching the ICU.
Additional anesthetic considerations for robotic adrenalectomy
All anesthetic considerations that apply to other robotic-assisted abdominal surgery similarly apply for robotic adrenalectomy. Muscle relaxation is especially important to ensure no damage to visceral structures occurs due to patient movement. Insufflation can be particularly stimulating during these cases. In patients without pheochromocytoma, increases in systemic vascular resistance, mean arterial pressure, and central venous pressure with an accompanying decrease in stroke volume and cardiac index are typically seen during insufflation of the abdomen [22]. However, the sympathetic surge caused from abdominal insufflation may often be exacerbated by catecholamine response from the pheochromocytoma resulting in profound hypertension and tachycardia [11].
Conclusion
In this article, we demonstrated a successful anesthetic and hemodynamic management for a robotic-assisted adrenalectomy for pheochromocytoma. We reviewed the current anesthesia considerations for perioperative management of a robotic-assisted pheochromocytoma resection. Patients must be carefully optimized in the preoperative period with diagnostic endocrinologic/cardiac workup and alpha blockade optimization. The surgical approach, whether minimally invasive or open, results in important hemodynamic changes to the anesthetic plan. To preclude hemodynamic instability, the anesthesiologist should be prepared with vasodilating, antiarrhythmic, and vasopressor medications for the different phases of surgery. The patient's postoperative disposition should be decided with input from the multidisciplinary treatment team.
Acknowledgments
The authors would like to acknowledge Dr. Renyu Liu who helped with the preparation of this manuscript.
References
- Golden SH, Robinson KA, Saldanha I, Anton B, Ladenson PW. Prevalence and Incidence of Endocrine and Metabolic Disorders in the United States: A Comprehensive Review. The Journal of Clinical Endocrinology & Metabolism. 2009; 94: 1853-1878. doi: 10.1210/jc.2008-2291
- Lafont M, Fagour C, Haissaguerre M, Darancette G, Wagner T, Corcuff JB, et al. Per-operative Hemodynamic Instability in Normotensive Patients With Incidentally Discovered Pheochromocytomas. The Journal of Clinical Endocrinology & Metabolism. 2015; 100: 417-421. doi: 10.1210/jc.2014-2998
- Roizen MF, Horrigan RW, Koike M, Eger EI, Mulroy MF, Frazer B, et al. A Prospective Randomized Trial of Four Anesthetic Techniques for Resection of Pheochromocytoma. Anesthesiology. 1982; 57. doi: 10.1097/00000542-198209001-00043
- Ramakrishna H. Pheochromocytoma resection: Current concepts in anesthetic management. Journal of Anaesthesiology Clinical Pharmacology. 2015; 31: 317. doi: 10.4103/0970-9185.161665
- Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2014; 130. doi: 10.1161/cir.0000000000000106
- Weingarten TN, Welch TL, Moore TL, Walters GF, Whipple JL, Cavalcante A, et al. Preoperative Levels of Catecholamines and Metanephrines and Intraoperative Hemodynamics of Patients Undergoing Pheochromocytoma and Paraganglioma Resection. Urology. 2017; 100: 131-138. doi: 10.1016/j.urology.2016.10.012
- Fang AM, Rosen J, Saidian A, Bae S, Tanno FY, Chambo JL, et al. Perioperative outcomes of laparoscopic, robotic, and open approaches to pheochromocytoma. Journal of Robotic Surgery. 2020; 14: 849-854. doi: 10.1007/s11701-020-01056-9
- Nomine-Criqui C, Germain A, Ayav A, Bresler L, Brunaud L. Robot-assisted adrenalectomy: Indications and drawbacks. Updates in Surgery. 2017; 69: 127-133. doi: 10.1007/s13304-017-0448-6
- Aliyev S, Karabulut K, Agcaoglu O, Wolf K, Mitchell J, Siperstein A, et al. Robotic Versus Laparoscopic Adrenalectomy for Pheochromocytoma. Annals of Surgical Oncology. 2013; 20: 4190-4194. doi: 10.1245/s10434-013-3134-z
- Ma W, Mao Y, Zhuo R, Dai J, Fang C, Wang C, et al. Surgical outcomes of a randomized controlled trial compared robotic versus laparoscopic adrenalectomy for pheochromocytoma. European Journal of Surgical Oncology. 2020; 46: 1843-1847. doi: 10.1016/j.ejso.2020.04.001
- Joris JL, Hamoir EE, Hartstein GM, Meurisse MR, Hubert BM, Charlier CJ, et al. Hemodynamic Changes and Catecholamine Release During Laparoscopic Adrenalectomy for Pheochromocytoma. Anesthesia & Analgesia. 1999; 88: 16-21. doi: 10.1097/00000539-199901000-00004
- Weingarten TN, Cata JP, Ohara JF, Prybilla DJ, Pike TL, Thompson GB, et al. Comparison of Two Preoperative Medical Management Strategies for Laparoscopic Resection of Pheochromocytoma. Urology. 2010; 76. doi: 10.1016/j.urology.2010.03.032
- Santos JRU, Brofferio A, Viana B, Pacak K. Catecholamine-Induced Cardiomyopathy in Pheochromocytoma: How to Manage a Rare Complication in a Rare Disease? Hormone and Metabolic Research. 2018; 51: 458-469. doi: 10.1055/a-0669-9556
- Naranjo J, Dodd S, Martin YN. Perioperative Management of Pheochromocytoma. Journal of Cardiothoracic and Vascular Anesthesia. 2017; 31: 1427-1439. doi: 10.1053/j.jvca.2017.02.023
- Castillo OA, Vitagliano G, Olivares R, Soffia P, Contreras M. Laparoscopic Resection of an Extra-adrenal Pheochromocytoma. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques. 2007; 17: 351-353. doi: 10.1097/sle.0b013e318059b9d4
- Siddiqi HK, Yang H-Y, Laird AM, Fox AC, Doherty GM, Miller BS, et al. Utility of oral nicardipine and magnesium sulfate infusion during preparation and resection of pheochromocytomas. Surgery. 2012; 152: 1027-1036. doi: 10.1016/j.surg.2012.08.023
- Erdogan MA, Ozkan AS, Ozgul U, Colak Y, Ucar M. Dexmedetomidine, Remifentanil, and Sevoflurane in the Perioperative Management of a Patient during a Laparoscopic Pheochromocytoma Resection. Journal of Cardiothoracic and Vascular Anesthesia. 2015; 29. doi: 10.1053/j.jvca.2015.06.024
- Desmonts J, Marty J. Anaesthetic Management of Patients With Phaeochromocytoma. British Journal of Anaesthesia. 1984; 56: 781-789. doi: 10.1093/bja/56.7.781
- Niederle MB, Fleischmann E, Kabon B, Niederle B. The determination of real fluid requirements in laparoscopic resection of pheochromocytoma using minimally invasive hemodynamic monitoring: a prospectively designed trial. Surgical Endoscopy. 2019; 34: 368-376. doi: 10.1007/s00464-019-06777-z
- Thompson JP, Bennett D, Hodson J, Asia M, Ayuk J, O'Reilly MW, et al. Incidence, risk factors and clinical significance of postoperative haemodynamic instability after adrenalectomy for phaeochromocytoma. Gland Surgery. 2019; 8: 729-739. doi: 10.21037/gs.2019.11.22
- Mamilla D, Araque K, Brofferio A, Gonzales M, Sullivan J, Nilubol N, et al. Postoperative Management in Patients with Pheochromocytoma and Paraganglioma. Cancers. 2019; 11: 936. doi: 10.3390/cancers11070936
- Mclaughlin J, Scheeres D, Dean R, Bonnell B. The adverse hemodynamic effects of laparoscopic cholecystectomy. Surgical Endoscopy. 1995; 9. doi: 10.1007/bf00191950