Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/163
Research Article | Volume 9 | Issue 4 Open Access
Intrathecal Administration of the Fat-Mass and Obesity-Associated Protein Inhibitor Mitigates Neuropathic Pain in Female Rats
Xiang Li1 and Yuan-Xiang Tao1,2,3*
1Department of Anesthesiology, New Jersey Medical School, Rutgers, The State University of New Jersey, Newark, USA
2Department of Physiology, Pharmacology & Neuroscience, New Jersey Medical School, Rutgers, The State University of New Jersey, Newark, USA
3Departments of Cell Biology & Molecular Medicine, New Jersey Medical School, Rutgers, The State University of New Jersey, Newark, USA
Dr. Yuan-Xiang Tao, Department of Anesthesiology, New Jersey Medical School, Rutgers, The State University of New Jersey, 185 S. Orange Ave., MSB, F-661, Newark, NJ 07103, USA, Tel: +1-973-972-9812; Fax: +1-973-972-1644, E-mail: yuanxiang.tao@njms.rutgers.eduEditor: Daqing Ma, MD, PhD, FRCA, Professor of Anaesthesia and BOC Chair, Head of Anaesthesia Research, Imperial College London, G3, 44 Chelsea & Westminster Hospital, 369 Fulham Road, London SW10 9NH
Received: October 29, 2022 | Accepted: November 28, 2022 | Published: November 30, 2022
Citation: Xiang L, Yuan-Xiang T. Intrathecal Administration of the Fat-Mass and Obesity-Associated Protein Inhibitor Mitigates Neuropathic Pain in Female Rats. Transl Perioper & Pain Med 2022; 9(4):478-487
Abstract
Several intracellular signals are involved in the sexual dimorphism of chronic pain. Our previous studies demonstrated that the fat-mass and obesity-associated protein (FTO), a demethylase of RNA N6-methyladenosine, in the injured dorsal root ganglion (DRG) contributed to the development and maintenance of nerve injury-induced nociceptive hypersensitivity in male rats and male mice. However, whether these functions of DRG FTO are in a sex-dependent manner is still unknown. The present study sought to investigate the effect of intrathecal administration of a specific FTO inhibitor, meclofenamic acid (MA), on chronic constriction injury (CCI)-induced nociceptive hypersensitivity in female rats. Intrathecal injection of MA attenuated the CCI-induced mechanical allodynia, heat hyperalgesia, and cold hyperalgesia in both induction and maintenance periods, without changing acute/basal pain and locomotor function, in female rats. Intrathecal MA also blocked the CCI-induced hyperactivations of neurons and astrocytes in the ipsilateral L4 and L5 dorsal horns of female rats. Mechanistically, intrathecal MA prevented the CCI-induced increase in the histone methyltransferase G9a expression and reversed the G9a-controlled downregulation of mu-opioid receptor and Kv1.2 proteins in the ipsilateral L4 and L5 DRGs of female rats. These findings indicate that the effects of the FTO inhibitor on nerve injury-induced nociceptive hypersensitivity in female rats are similar to those in male rats reported previously. Our data also further confirm the role of DRG FTO in neuropathic pain and suggest potential clinical application of the FTO inhibitors for the prevention and treatment of this disorder in both men and women.
Keywords
Fat-mass and obesity-associated protein, Meclofenamic acid, Intrathecal injection, Histone methyltransferase G9a, mu opioid receptor, Kv1.2, Dorsal root ganglion, Neuropathic pain, Female rats
Introduction
Chronic neuropathic pain, a major public health crisis, negatively influences the quality of life of approximately 6.9% to 14% of the population in the world [1]. About 600 billion dollars annually are costed on neuropathic pain-related healthcare and productivity losses in the United States [2]. Therapeutic management for this debilitating disorder is highly limited in success, as current approaches including pharmacological drugs (e.g., opioids, antidepressants and anticonvulsants) do not produce sufficient pain relief in most neuropathic pain patients [3]. Neuropathic pain in clinic is characterized by spontaneous ongoing pain, allodynia (pain due to innocuous stimuli) and hyperalgesia (augmented pain from noxious stimuli). The generation of these pain hypersensitivities is believed to be related to nerve injury-induced dysregulation of pain-associated genes at both transcriptional and translational levels in the sensory nervous system [4-9]. Thus, elucidating the mechanisms by which these genes are dysregulated following nerve injury may open a new door for neuropathic pain management.
N6-methyladenosine (m6A) modification of RNA contributes to neuropathic pain genesis by regulating the expression of pain-associated genes in the dorsal root ganglion (DRG) [10-12]. The fat-mass and obesity-associated protein (FTO), a demethylase, erases m6A in mRNA [13]. FTO was expressed exclusively in the neurons of DRG [11]. The levels of Fto mRNA and FTO protein were time-dependently increased in the injured DRG following peripheral nerve injury [11]. Blocking this increase through DRG microinjection of Fto siRNA or AAV5 expressing the FTO-specific guide RNA in male rats or DRG microinjection of AAV5 expressing Cre in male Ftofl/fl mice reversed a loss of m6A sites in euchromatic histone methyltransferase 2 (Ehmt2) mRNA (encoding the histone methyltransferase G9a) and destabilized the nerve injury-induced G9a upregulation in the injury DRG and mitigated nerve injury-induced nociceptive hypersensitivities [11]. Mimicking nerve injury-induced increase of DRG FTO through DRG microinjection of AAV5 expressing full-length Fto erased m6A in Ehmt2 mRNA and elevated G9a level in microinjected DRGs and led to enhanced responses to mechanical, heat and cold stimuli in naive male rats [11]. Given that G9a is a key player in the induction and maintenance of neuropathic pain [6,14-16], FTO likely participates in neuropathic pain through stabilizing nerve injury-induced upregulation of G9a in primary sensory neurons [10]. More importantly, intrathecal injection of two specific FTO inhibitors, meclofenamic acid (MA) and N-CDPCB, significantly blocked nerve injury-induced mechanical allodynia, heat hyperalgesia and spontaneous ongoing nociceptive responses in both the development and maintenance periods in male rats [12]. Given that MA is an FDA-approved nonsteroidal anti-inflammatory drug [17,18], these findings strongly suggest that FTO inhibitors may have potential applications for neuropathic pain in the clinical setting.
Previous studies have demonstrated that several intracellular signals are involved in the sexual dimorphism of pain. IL-23 augmented C-fiber-mediated and blue light-induced spontaneous pain in female, but not male, mice [19,20]. Resolvins D5 repressed neuropathic and inflammatory pain in male, but not female, mice [21]. TLR4 mediated the development of inflammatory and neuropathic pain in male, but not female, mice [22-24]. Whether FTO is involved in the sexual dimorphism of neuropathic pain is unknown. In the present study, we observed whether the effect of intrathecal administration of the specific FTO inhibitor MA on nerve injury-induced nociceptive hypersensitivity in female rats was similar to that in male rats reported in our previous study [12].
Materials and Methods
Animal preparation
Female Sprague-Dawley rats weighing 180-250 g were purchased from Charles River Laboratories and were kept under a 12-h light-dark cycle with ad-libitum food and water. All animal experimental procedures were coordinated with ethical guidelines issued by the National Institutes of Health and the International Association for the Study of Pain. The protocols were approved by the Rutgers New Jersey Medical School Animal Care and Use Committee. All efforts were committed to minimize the number of animals used and any subsequent suffering involved. To reduce the individual variability in behavioral outcome measurements, animals were randomly assigned to experimental groups (n = 6 rats/group) and acclimated for 1-2 days before behavioral testing. All the experimenters were blind to the treatment condition.
Neuropathic pain model
A preclinical rat model of CCI-induced neuropathic pain was carried out as described previously [25-28]. In brief, after the rats were anesthetized with 2% isoflurane, the unilateral sciatic nerve was exposed and loosely ligated with 4-0 silk thread at three sites with an interval of about 1mm proximal to trifurcation of the sciatic nerve. Sham rats were subjected to sciatic nerve exposures and isolation without ligation.
Intrathecal catheterization and drug delivery
Intrathecal catheterization was performed following the procedures described previously [29-31]. Briefly, the longitudinal incision was made over the spinous processes of L4 and L5 vertebrae after rats were completely anesthetized and maintained with 2% isoflurane. The fascia and superficial muscles around the spinous processes were dissected to expose intervertebral space. After the dura was discreetly punctured by a 22-gauge needle, a polyethylene-10 (PE-10) catheter was implanted into the subarachnoid space. After the residual of the catheter was fastened to the surrounding muscles, a subcutaneous dorsal tunnel to the neck area was performed. The location of the catheter was validated by administrating 10 μl of 2% lidocaine 2 days after the operation. The animals that showed complete paralysis of the tail and bilateral hind legs within 30 s and complete recovery within 30 min after lidocaine injection were deemed to be successfully modeled. These animals without any locomotor deficit or poor grooming habits 7 days after catheter implantation were used for further experiments.
The MA (Millipore-Sigma, St. Louis, MO) was dissolved in 0.01M phosphate-buffered saline (PBS) or vehicle (0.01M PBS) and was intrathecally injected 30 min before surgery and once daily for 5 consecutive days after surgery, or once daily for 5 days starting on postoperative day 9. The volume of intrathecal injection for MTA or vehicle was 10 μl in total followed by 12 μl vehicle to flush the catheter. The dosage of the drug used was based on our previous study on male rats [12].
Behavioral tests
Mechanical, heat, and cold tests as well as locomotor function tests were performed as described previously [8,9,30,32-34]. The one-hour interval was given between two tests.
The paw withdrawal threshold was defined as the hind paw withdrawal response to von Frey filament stimuli (Stoelting Co., Wood Dale, IL) by using the up-and-down method. Briefly, an individual rat was placed in a Plexiglas chamber on an elevated mesh screen. Von Frey filaments in log increments of force (0.407, 0.692, 1.202, 2.041, 3.63, 5.495, 8.511, 15.14, 26.0g) were applied to the plantar surface of the ipsilateral and contralateral hind paws, starting from the 3.63 g von Frey filament. A positive response was determined as a quick paw withdrawal with or without shaking. When a positive response was observed, the next lower force filament was applied. On the contrary, when a negative response occurred, the next higher force filament was used. The test was ended under either one of two following two situations: 1) A negative response was achieved with the highest force (26.0 g) and 2) Three applications were proceeded following the first positive response. The pattern of response was converted into a 50% von Frey threshold by using the formula described previously [35,36].
Paw withdrawal latency to noxious heat stimulation was measured by using a Model 336 Analgesic Meter (IITC Inc./Life Science Instruments, Woodland Hills, CA). In brief, an individual rat was placed in a Plexiglas chamber on a glass plate. A beam of light from the Model 336 Analgesic Meter was applied to the middle of the plantar surface of each hind paw through the glass plate. The light was automatically turned off when the paw was lifted or withdrawn. The paw withdrawal latency was calculated as the time between the beginning of the light beam and paw withdrawal/lift. Each trial was repeated 3 times at 5 min intervals for each side. To avoid paw tissue damage, the cutoff time was set as 20s.
Paw withdrawal latency to noxious cold (0 °C) was assessed by using a cold aluminum plate, which the temperature was monitored constantly via a digital thermometer. The paw withdrawal latency was defined as the time between the placement of the rat on the plate and a quick paw jump with or without the paw flinching/shaking. Each trial was repeated 3 times at 10 min intervals. The cutoff time was set as 60s to prevent paw damage.
The locomotor function test was carried out after the above-described behavioral tests and included the following three reflexes. Placing reflex: The hind limbs were placed slightly lower than the forelimbs and the dorsal surfaces of the hind paws were brought into contact with the edge of a table. Whether the hind paws were placed on the table surface reflexively or not was recorded. Grasping reflex: After the rat was placed on a wire mesh, whether the hind paws grasped the wire on contact or not was recorded. Righting reflex: When the rat was placed on its back on a flat surface, whether it immediately assumed the normal upright position or not was recorded. Each trial was repeated 5 times at 5 min intervals and the scores for placing, grasping, and righting reflexes were recorded according to the counts of each normal reflex displayed in five trials. Additionally, the animal's general behaviors, including spontaneous activity were also observed.
Western blot analysis
Western blot analysis was carried out as described previously [37-41]. In brief, the ipsilateral L4/5 DRGs and spinal cord were collected and stored at a -80 °C freezer. The samples were homogenized in chilled lysis buffer (10 mM Tris, 5 mM MgCl2, 5 mM EGTA, 1 mM EDTA, 250 mM sucrose, 1 mM phenylmethylsulfonyl fluoride, 1 mM DTT, 40 μM leupeptin, and 1% phosphatase inhibitor cocktail II and III). After the crude homogenate was centrifuged at 4 °C for 15 min at 1,000 × g, the supernatants were collected for cytosolic proteins and the pellets for nuclear proteins. The pellets were dissolved in the lysis buffer plus 1.5% SDS and 0.3% Triton-X 100. After protein concentration was measured, the samples were heated at 99 °C for 5 min and loaded onto a 4%-20% precast polyacrylamide gel (Bio-Rad Laboratories). The proteins were then electrophoretically transferred onto 0.2 µm pore-size nitrocellulose membranes (Bio-Rad Laboratories). The membranes were blocked with 3% non-fat milk in Tris-buffered saline containing 0.1% Tween-20 for 1 h and then incubated at 4 °C overnight with the following primary antibodies. These antibodies included mouse anti-FTO (1:1,000, Abcam, Cambridge, MA), rabbit anti-G9a (1:800, Abcam), mouse anti-Kv1.2 (1:800, NeuroMab, Davis, CA), rabbit anti-MOR (1:1,000, ImmunoStar, Husson, WI), rabbit anti-phosphorylated-ERK1/2 (p-ERK1/2; Thr202/Tyr204, 1:1,000, Cell Signaling, Danvers, MA), rabbit anti-ERK1/2 (1:1,000, Cell Signaling), mouse anti-GFAP (1:1,000, Cell Signaling), rabbit anti-GAPDH (1:2,000, Santa Cruz, Dallas, TX), or rabbit anti-histone H3 (1:2,000, Cell Signaling). The proteins were detected by horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibody (1:3,000, Jackson ImmunoResearch, West Grove, PA), developed by Western peroxide reagent and luminol/enhancer reagent (Clarity Western ECL Substrate, Bio-Rad, Hercules, CA), and visualized by ChemiDoc XRS and System with Image Lab software (Bio-Rad). The intensity of blotting was quantified with densitometry using Image Lab software (Bio-Rad). The relative density values from the remaining treated groups were determined by dividing the optical density values from these groups by the average value of the vehicle plus sham groups after each was normalized to the corresponding histone H3 (for nucleus proteins) or GAPDH (for cytosolic proteins).
Statistical analysis
The female rats were randomly assigned into various treated groups. All results were expressed as mean ± S.E.M. All data were statistically analysed by using a two-way or three-way analysis of variants (ANOVA) with repeated measures followed by the post hoc Tukey method (Sigma Plot 12.5, San Jose, CA). Significance was set at P < 0.05.
Results
Effect of intrathecal MA on the development of CCI-induced nociceptive hypersensitivity in female rats
Our previous study showed that intrathecal administration of the specific FTO inhibitors significantly mitigated the development of SNL-induced nociceptive hypersensitivity in male rats [12]. To examine whether the FTO inhibitor had the similar effect in the female rats, we intrathecally administered MA at 20 µg 30 min before CCI or sham surgery and once daily for 5 consecutive days. In line with previous reports [25-28], CCI led to long-lasting mechanical allodynia, heat hyperalgesia and cold hyperalgesia on the ipsilateral, but not contralateral, side in the vehicle-treated CCI female rats (Figure 1A, Figure 1B, Figure 1C, Figure 1D and Figure 1E). On days 3 and 5 post-CCI, paw withdrawal thresholds in response to von Frey filament stimuli and paw withdrawal latencies in response to heat and cold stimuli were significantly reduced as compared to those in the vehicle-treated sham rats (Figure 1A, Figure 1B and Figure 1C). However, these nociceptive hypersensitivities were alleviated in the MA-treated CCI female rats (Figure 1A, Figure 1B and Figure 1C). Paw withdrawal thresholds to mechanical stimulation were increased by 82% and 108% on days 3 and 5, respectively, post-CCI (Figure 1A) and paw withdrawal latencies to heat and cold stimuli were increased by 39% and 30%, respectively, on day 3 post-CCI and by 49% and 39%, respectively, on day 5 post-CCI as compared to the vehicle-treated CCI rats at the corresponding time points (Figure 1B and Figure 1C). As expected, MA at the dosage used did not change basal paw withdrawal responses to mechanical, heat and cold stimuli on the contralateral side of CCI rats and on both ipsilateral and contralateral sides of sham rats during the observation period (Figure 1A, Figure 1B, Figure 1C, Figure 1D and Figure 1E).
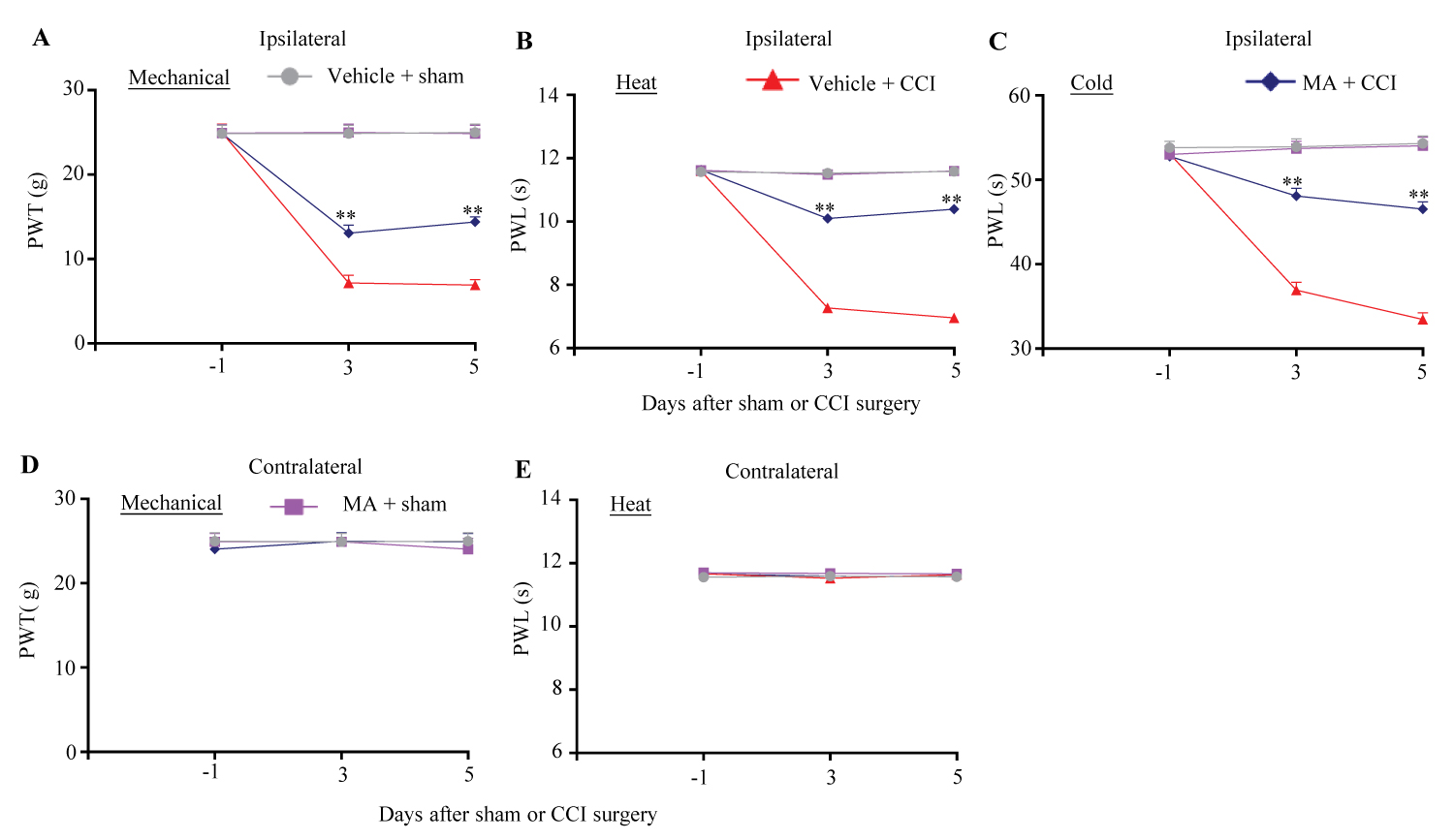
Figure 1:
Figure 1: Effect of intrathecal pre-administration of meclofenamic acid (MA) on the development of CCI-induced nociceptive hypersensitivity in female rats. 20 μg MA (dissolved in 0.01 M PBS) or vehicle (0.01 M PBS) was intrathecally (i.th) administrated starting 30 min before CCI or sham surgery and once daily for 5 days after surgery. Paw withdrawal threshold (PTW) to mechanical stimulation (A and D) and paw withdrawal latency (PWL) to heat (B and E) and cold (C) stimuli on the ipsilateral (A-C) and contralateral (D and E) sides were carried out one day before surgery and on days 3 and 5 post-surgery. n = 6 female rats/group. Three-way ANOVA with repeated measures followed by post hoc Tukey test.
**P < 0.01 versus the vehicle-treated CCI female rats at the corresponding time points.
Effect of intrathecal MA on the maintenance of CCI-induced nociceptive hypersensitivity in female rats
The role of FTO inhibitor in the maintenance of CCI-induced nociceptive hypersensitivity in female rats was also examined. MA at 20 µg was intrathecally injected once daily for 5 consecutive days beginning on day 9 post-surgery, at this point, CCI-induced mechanical allodynia, heat hyperalgesia and cold hyperalgesia were fully developed [25-28]. Intrathecal MA produced the increases in paw withdrawal threshold to mechanical stimuli by 97% and 92% on day 12 and 14 post-CCI, respectively, (Figure 2A) and in paw withdrawal latencies to heat and cold stimuli by 43% and 35%, respectively, on day 12 post-CCI and by 40% and 31%, respectively, on day 14 post-CCI, as compared to those in the vehicle-treated CCI rats at the corresponding time points (Figure 2B and Figure 2C). Expectedly, intrathecal MA did not alter basal responses to mechanical, heat and cold stimuli on the contralateral side of CCI female rats and on both sides of sham female rats on days 12 and 14 post-surgery (Figure 2A, Figure 2B, Figure 2C, Figure 2D and Figure 2E).
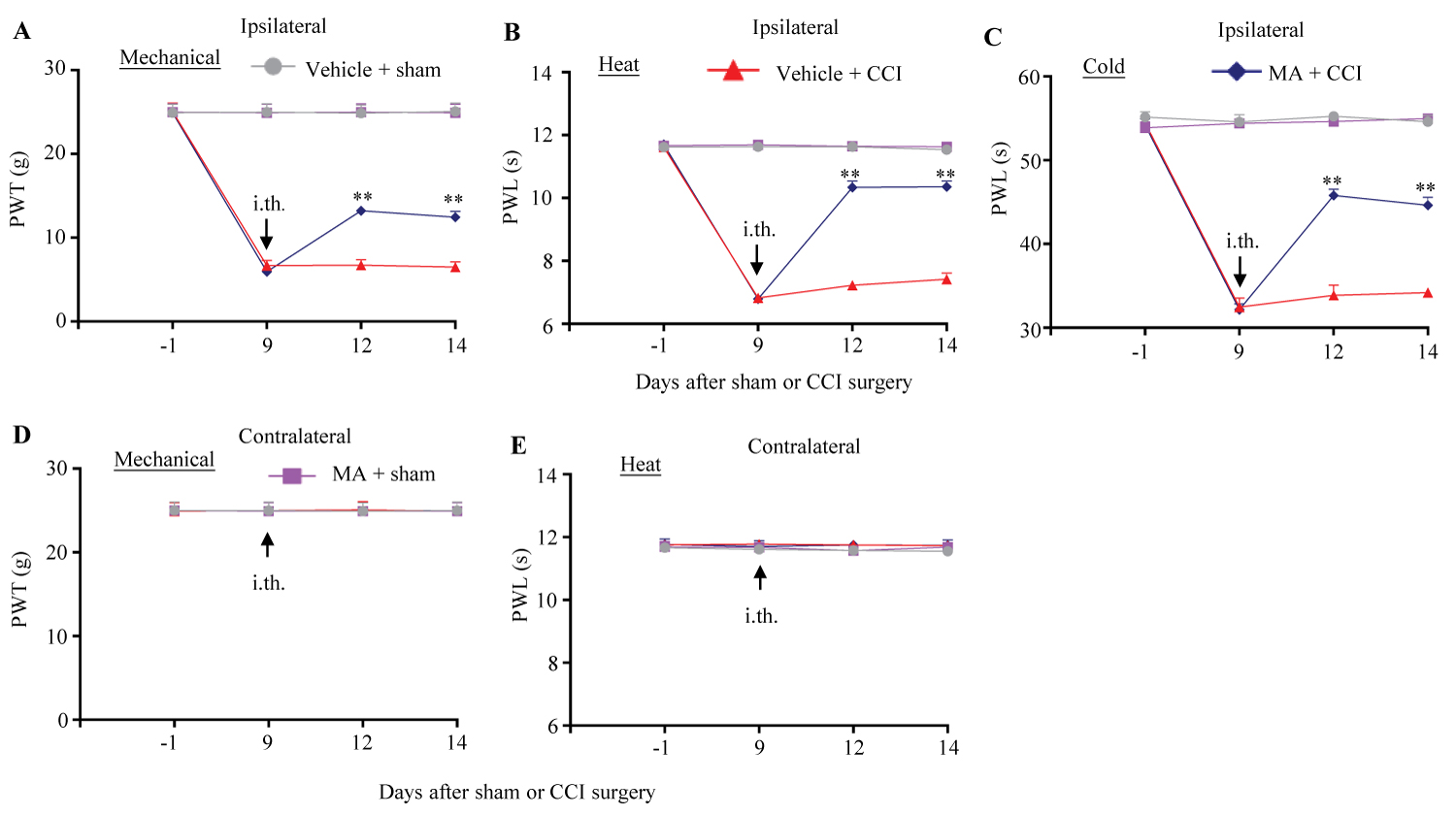
Figure 2:
Figure 2: Effect of intrathecal post-administration of meclofenamic acid (MA) on the maintenance of CCI-induced nociceptive hypersensitivity in female rats. 20 μg MA or vehicle (0.01 M PBS) was intrathecally (i.th.) administrated once daily for 5 days starting on day 9 post-surgery. Paw withdrawal threshold (PTW) to mechanical stimulation (A and D) and paw withdrawal latency (PWL) to heat (B and E) and cold (C) stimuli on the ipsilateral (A-C) and contralateral (D and E) sides were performed one day before surgery, prior to MA or vehicle administration on day 9 post-surgery and on days 12 and 14 post-surgery. n = 6 female rats/group. Three-way ANOVA with repeated measures followed by post hoc Tukey test.
**P < 0.01 versus the vehicle-treated CCI female rats at the corresponding time points.
Effect of intrathecal MA on the CCI-induced neuronal and astrocyte hyperactivation in spinal cord dorsal horn of female rats
In the dorsal horn, peripheral nerve injury triggered neuronal and astrocyte hyperactivation evidenced by the increases in the levels of phosphorylation of extracellular signal-regulated kinase 1/2 (p-ERK1/2) and glial fibrillary acidic protein (GFAP), respectively [25-28]. To further confirm the effect of intrathecal MA on CCI-induced behaviors, we observed whether intrathecal MA at 20 µg impacted the CCI-induced increases of p-ERK1/2 and GFAP in the dorsal horn. In line with previous studies [25-28], the amounts of p-ERK1/2 and GFAP were remarkably increased in the ipsilateral L4/5 dorsal horn of vehicle-treated CCI female rats on day 14 post-surgery (Figure 3A and Figure 3B). These increases were abolished in the MA-treated CCI female rats (Figure 3A and Figure 3B). Intrathecal MA did not alter the basal expression of p-ERK1/2 or GFAP the ipsilateral L4/5 dorsal horn of sham female rats and of total ERK1/2 in either MA- or vehicle-treated CCI/sham female rats (Figure 3A and Figure 3B).
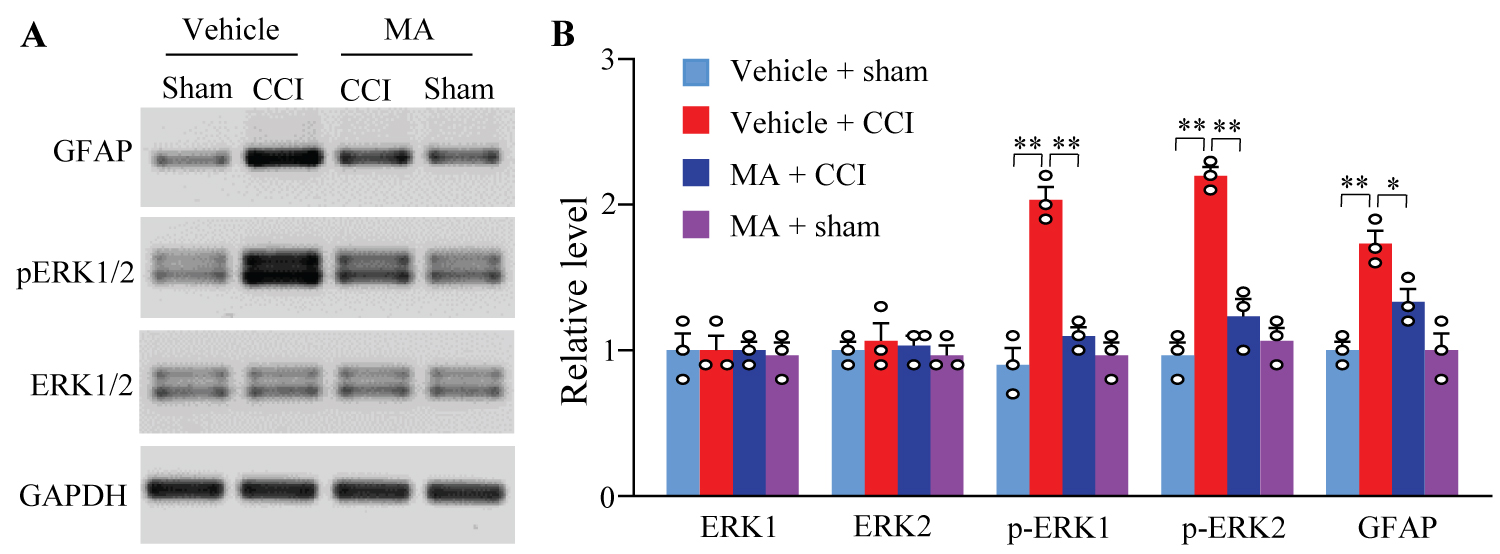
Figure 3:
Figure 3: Effect of intrathecal post-administration of meclofenamic acid (MA) on the CCI-induced hyperactivation in spinal cord dorsal horn neurons and astrocytes on the ipsilateral side in female rats. Levels of phosphorylated extracellular signal-regulated kinase 1 and 2 (p-ERK1/2, a marker of neuronal hyperactivation), total ERK1/2, and glial fibrillary acidic protein (GFAP, a marker of astrocyte hyperactivation) in the ipsilateral lumbar 4/5 dorsal horn on day 14 post-CCI or sham surgery. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was set as a loading control. Representative Western blots (A) and a summary of densitometric analysis (B). n = 3 biological repeats (3 rats)/group.
*P<0.05 or **P < 0.01 by two-way ANOVA followed by post hoc Tukey test.
Effect of intrathecal MA on the CCI-induced increase of G9a and decrease of Mu opioid receptor and Kv1.2 in injured DRG of female rats
We examined whether intrathecal MA affected the FTO-mediated downstream signal G9a and G9a-controlled mu-opioid receptor (MOR) and Kv1.2 in injured DRG of female rats following CCI. Consistent with the previous work [14,15], CCI produced significant increases in the levels of G9a two isoforms (short and long) and reductions in the amounts of MOR and Kv1.2 in the ipsilateral L4/5 DRGs on day 14 post-CCI in the vehicle-treated rats (Figure 4A and Figure 4B). These alternations were markedly blocked by intrathecal MA at 20 µg (Figure 4A and Figure 4B). As expected, intrathecal MA did not impact the CCI-induced increase in the level of FTO in the ipsilateral L4/5 DRGs on day 14 post-CCI and basal levels of FTO, G9a two isoforms, MOR and Kv1.2 in the ipsilateral L4/5 DRGs on day 14 post-sham surgery (Figure 4A and Figure 4B).
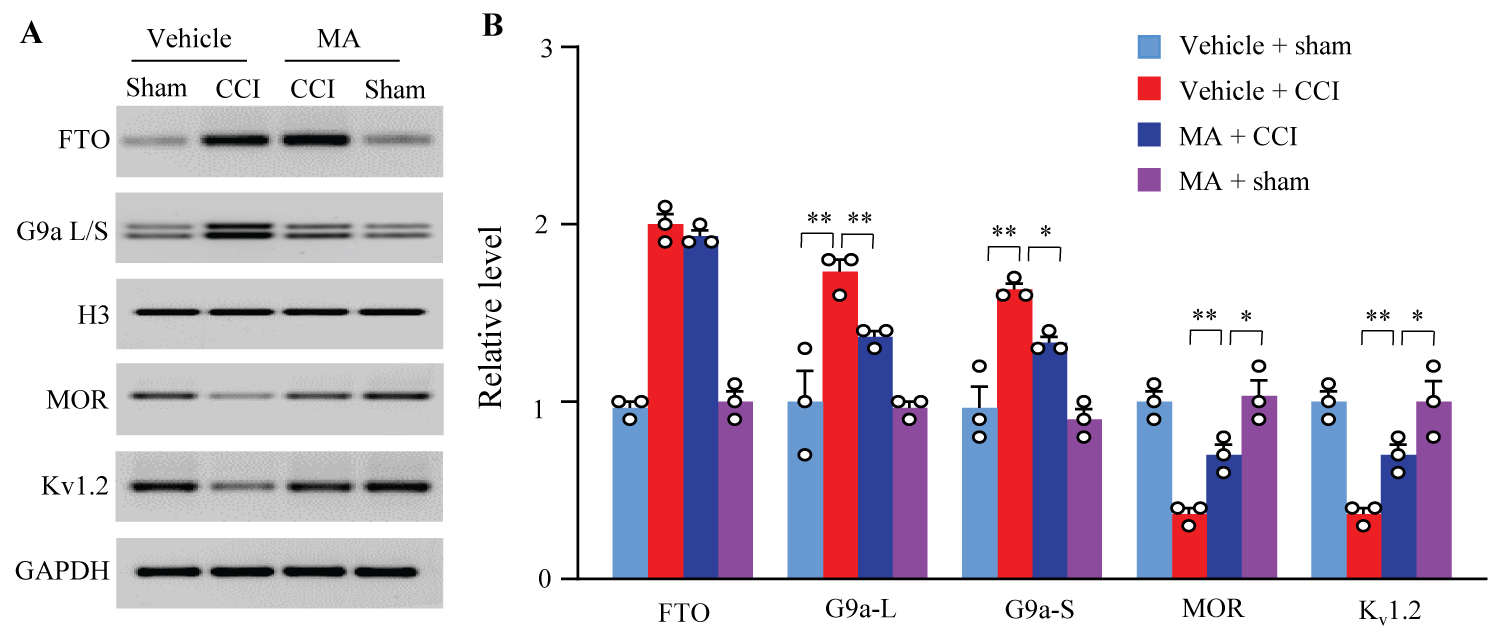
Figure 4:
Figure 4: Effect of intrathecal post-administration of meclofenamic acid (MA) on the CCI-induced upregulation of G9a and downregulation of mu-opioid receptor (MOR) and Kv1.2 in the injured DRGs. L: Long isoform. S: Short isoform. Levels of FTO, two isoforms of G9a, MOR, and Kv1.2 proteins in the ipsilateral lumbar 4/5 DRGs on day 14 post-CCI or sham surgery. H3 was set as a loading control for nuclear fraction and GAPDH for cytosolic/membrane fraction. Representative Western blots (A) and a summary of densitometric analysis (B). n = 3 biological repeats (6 rats)/group.
*P<0.05 or **P < 0.01 by two-way ANOVA followed by post hoc Tukey test.
Effect of intrathecal MA on locomotor function in female rats
To exclude the possibility that MA antinociceptive effect was attributed to impairing locomotor activity, we finally examined locomotor activities including grasping, placing, and righting reflexes in the injected female rats. Either MA- or vehicle-treated female rats exhibited normal placing, grasping and righting reflexes post-CCI or sham surgery (Table 1). Additionally, no treated female rats displayed convulsions and hypermobility. General behaviors including gait and spontaneous activity are normal in all treated female rats.
Table 1: Locomotor function.
|
Treated groups |
Placing |
Grasping |
Righting |
|
Sham + vehicle |
5 (0) |
5 (0) |
5 (0) |
|
Sham + MA |
5 (0) |
5 (0) |
5 (0) |
|
CCI + vehicle |
5 (0) |
5 (0) |
5 (0) |
|
CCI + MA |
5 (0) |
5 (0) |
5 (0) |
MA: Meclofenamic acid. n = 6 rats per group. 5 trials. Mean (SEM)
Discussion
The present work proved that intrathecal administration of the FTO inhibitor MA significantly alleviated the induction and maintenance of the CCI-induced mechanical allodynia, heat hyperalgesia and cold hyperalgesia and blocked the CCI-induced hyperactivation of neurons and astrocytes in spinal cord dorsal horn in the female rats. This administration also attenuated the CCI-induced increase in G9a expression and reversed the CCI-induced downregulation of MOR and Kv1.2 in the injured DRG of female rats. It appears that MA effects on nerve injury-induced nociceptive hypersensitivity in female rats are similar to those in male rats reported previously [12]. Our findings also further confirmed an important role of DRG FTO in the peripheral mechanisms underlying neuropathic pain.
Previous studies have demonstrated that several intracellular signals participate in chronic pain including neuropathic pain in a sex-dependent manner. An early report showed that spinal microglial TLR4 signaling mediated the lipopolysaccharide-induced tactile allodynia, the complete Freund's adjuvant-induced inflammatory pain and spared nerve injury-induced neuropathic pain in male, but not female, mice [22]. This phenomenon was further confirmed by subsequent studies from other groups [23,24,42]. IL-23 in macrophage signaling induced significantly greater p38 phosphorylation, a marker of nociceptor activation, in DRGs of female than male mice and enhanced C-fiber-mediated and blue light-induced spontaneous pain in female, but not male, mice [19,20]. Resolvins D5 repressed neuropathic and inflammatory pain in male, but not female, mice likely through targeting immune cells (e.g., macrophages) in DRG [21]. These studies may uncover the role of neuroimmune interaction to mediate pain states in a sex-specific fashion. Interestingly, recent works showed that the deletion of TLR4 protected both male and female mice from the development of cisplatin-induced polyneuropathy [43]. TLR4 in sensory neurons mediated the induction of nerve injury-induced mechanical hypersensitivity in female mice [44]. These findings suggest that it is necessary to discern sex-specific differences in distinct cell types and various models of chronic pain. The present study demonstrated that intrathecal administration of the FTO inhibitor produced a similar antinociceptive effect on nerve injury-induced neuropathic pain in female rats as compared to that in male rats reported in our previous study [12]. However, whether the role of FTO in other chronic pain models has sexual dimorphism is unknown and remains to be further investigated.
FTO in the injured DRG neurons plays a key role in the induction and maintenance of neuropathic pain. FTO is expressed in DRG neurons [11]. Peripheral nerve injury upregulated the expression of Fto mRNA and FTO protein in the injured DRG of male rats and male mice [11]. Consistently, the present study revealed an increase in the level of FTO protein in the ipsilateral L4/5 DRGs of female rats following CCI. Blocking the nerve injury-induced FTO upregulation in the injured DRG rescued the nerve injury-induced m6A loss in Ehmt2 mRNA, attenuated the nerve injury-induced increase of G9a and reversed the G9a-controlled reductions of MOR and Kv1.2 in the injured DRG of male rats and male mice [11]. Blocking this upregulation also alleviated nerve injury-induced nociceptive hypersensitivity during the development and maintenance periods in male rats and male mice [11]. Moreover, intrathecal administration of specific FTO inhibitors not only blocked the spinal nerve ligation (SNL)-induced increase of G9a and rescued the SNL-induced reductions of MOR and Kv1.2 in the ipsilateral L5 DRG but also mitigated the SNL-induced the development and maintenance of nociceptive hypersensitivity in male rats [12]. Similar results were seen in the female rats after CCI in the present study. Given that G9a is the critical instigator in neuropathic pain genesis [10-12], our findings indicate that DRG FTO contributes to neuropathic pain through stabilizing nerve injury-induced G9a increase in the primary sensory neurons in a sex-independent manner.
Conclusions
The current study proved that intrathecal administration of the FTO inhibitor MA produced an anti-nociceptive effect during both development and maintenance periods of CCI-induced nociceptive hypersensitivity, without altering acute/basal pain and locomotor activity, in female rats. These effects were similar to those in male rats after SNL reported previously [12]. Given MA is an FDA-approved nonsteroidal anti-inflammatory drug [17,18], our findings strongly suggest that the FTO inhibitors may have clinical application for neuropathic pain managements.
Acknowledgements
This work was supported by the grants (R01NS111553 and RFNS113881) from the National Institutes of Health (Bethesda, Maryland, USA).
Authors' Contributions
Y.X.T. conceived the project and supervised all experiments. X.L. and Y.X.T. designed the project. X.L. performed the animal model and intrathecal injection, conducted behavioral experiments, carried out Western blot experiments and analyzed the data. X.L. wrote the draft of the manuscript. Y.X.T. edited and approved the manuscript.
Competing Interests
The authors declare no conflict of interests.
References
- Pasero C (2004) Pathophysiology of neuropathic pain. Pain Manag Nurs 5: 3-8. S1524904204001018 [pii];10.1016/j.pmn.2004.10.002 [doi].
- Gilron I, Baron R, Jensen T (2015) Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc 90: 532-545. S0025-6196(15)00105-6 [pii];10.1016/j.mayocp.2015.01.018 [doi].
- O'Connor AB (2009) Neuropathic pain: quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics 27: 95-112. 2 [pii].
- Liang L, Lutz BM, Bekker A, Tao YX (2015) Epigenetic regulation of chronic pain. Epigenomics 7: 235-245. 10.2217/epi.14.75 [doi].
- Lutz BM, Bekker A, Tao YX (2014) Noncoding RNAs: new players in chronic pain. Anesthesiology 121: 409-417. 10.1097/ALN.0000000000000265 [doi].
- Pan Z, Du S, Wang K, Guo X, Mao Q, Feng X, Huang L, Wu S, Hou B, Chang YJ, Liu T, Chen T, Li H, Bachmann T, Bekker A, Hu H, Tao YX (2021) Downregulation of a Dorsal Root Ganglion-Specifically Enriched Long Noncoding RNA is Required for Neuropathic Pain by Negatively Regulating RALY-Triggered Ehmt2 Expression. Adv Sci (Weinh ) 8: e2004515. 10.1002/advs.202004515 [doi].
- Wu S, Bono J, Tao YX (2019) Long noncoding RNA (lncRNA): A target in neuropathic pain. Expert Opin Ther Targets 23: 15-20. 10.1080/14728222.2019.1550075 [doi].
- Zhao JY, Liang L, Gu X, Li Z, Wu S, Sun L, Atianjoh FE, Feng J, Mo K, Jia S, Lutz BM, Bekker A, Nestler EJ, Tao YX (2017) DNA methyltransferase DNMT3a contributes to neuropathic pain by repressing Kcna2 in primary afferent neurons. Nat Commun 8: 14712. ncomms14712 [pii];10.1038/ncomms14712 [doi].
- Zhao X, Tang Z, Zhang H, Atianjoh FE, Zhao JY, Liang L, Wang W, Guan X, Kao SC, Tiwari V, Gao YJ, Hoffman PN, Cui H, Li M, Dong X, Tao YX (2013) A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat Neurosci 16: 1024-1031. nn.3438 [pii];10.1038/nn.3438 [doi].
- Albik S, Tao YX (2021) Emerging role of RNA m6A modification in chronic Pain 162:1897-1898. 10.1097/j. pain.0000000000002219 [doi];00006396-900000000-98137 [pii].
- Li Y, Guo X, Sun L, Xiao J, Su S, Du S, Li Z, Wu S, Liu W, Mo K, Xia S, Chang YJ, Denis D, Tao YX (2020) N(6)-Methyladenosine Demethylase FTO Contributes to Neuropathic Pain by Stabilizing G9a Expression in Primary Sensory Neurons. Adv Sci (Weinh ) 7: 1902402. 10.1002/advs.201902402 [doi];ADVS1771 [pii].
- Zheng BX, Guo X, Albik S, Eloy J, Tao YX (2021) Effect of Pharmacological Inhibition of Fat-Mass and Obesity-Associated Protein on Nerve Trauma-Induced Pain Hypersensitivities. Neurotherapeutics 18: 1995-2007. 10.1007/s13311-021-01053-2 [doi];10.1007/s13311-021-01053-2 [pii].
- Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG, He C (2011) N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol 7: 885-887. nchembio.687 [pii];10.1038/nchembio.687 [doi].
- Liang L, Gu X, Zhao JY, Wu S, Miao X, Xiao J, Mo K, Zhang J, Lutz BM, Bekker A, Tao YX (2016) G9a participates in nerve injury-induced Kcna2 downregulation in primary sensory neurons. Sci Rep 6: 37704.
- Liang L, Zhao JY, Gu X, Wu S, Mo K, Xiong M, Bekker A, Tao YX (2016) G9a inhibits CREB-triggered expression of mu opioid receptor in primary sensory neurons following peripheral nerve injury. Mol Pain 12: 1-16.
- Liang L, Zhao JY, Kathryn T, Bekker A, Tao YX (2019) BIX01294, a G9a inhibitor, alleviates nerve injury-induced pain hypersensitivities during both development and maintenance periods. Transl Perioper Pain Med 6: 106-114. 10.31480/2330-4871/097 [doi].
- Chen B, Li Y, Song R, Xue C, Xu F (2019) Functions of RNA N6-methyladenosine modification in cancer progression. Mol Biol Rep 46: 1383-1391. 10.1007/s11033-018-4471-6 [doi];10.1007/s11033-018-4471-6 [pii].
- Huang Y, Yan J, Li Q, Li J, Gong S, Zhou H, Gan J, Jiang H, Jia GF, Luo C, Yang CG (2015) Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res 43: 373-384. gku1276 [pii];10.1093/nar/gku1276 [doi].
- Ji J, He Q, Luo X, Bang S, Matsuoka Y, McGinnis A, Nackley AG, Ji RR (2021) IL-23 Enhances C-Fiber-Mediated and Blue Light-Induced Spontaneous Pain in Female Mice. Front Immunol 12: 787565. 10.3389/fimmu.2021.787565 [doi].
- Luo X, Chen O, Wang Z, Bang S, Ji J, Lee SH, Huh Y, Furutani K, He Q, Tao X, Ko MC, Bortsov A, Donnelly CR, Chen Y, Nackley A, Berta T, Ji RR (2021) IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice. Neuron 109: 2691-2706. S0896-6273(21)00456-6 [pii];10.1016/j.neuron.2021.06.015 [doi].
- Luo X, Gu Y, Tao X, Serhan CN, Ji RR (2019) Resolvin D5 Inhibits Neuropathic and Inflammatory Pain in Male But Not Female Mice: Distinct Actions of D-Series Resolvins in Chemotherapy-Induced Peripheral Neuropathy. Front Pharmacol 10: 745. 10.3389/fphar.2019.00745 [doi].
- Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, Salter MW, Mogil JS (2011) Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci 31: 15450-15454. 31/43/15450 [pii];10.1523/JNEUROSCI.3859-11.2011 [doi].
- Stokes JA, Cheung J, Eddinger K, Corr M, Yaksh TL (2013) Toll-like receptor signaling adapter proteins govern spread of neuropathic pain and recovery following nerve injury in male mice. J Neuroinflammation 10: 148. 1742-2094-10-148 [pii];10.1186/1742-2094-10-148 [doi].
- Woller SA, Ravula SB, Tucci FC, Beaton G, Corr M, Isseroff RR, Soulika AM, Chigbrow M, Eddinger KA, Yaksh TL (2016) Systemic TAK-242 prevents intrathecal LPS evoked hyperalgesia in male, but not female mice and prevents delayed allodynia following intraplantar formalin in both male and female mice: The role of TLR4 in the evolution of a persistent pain state. Brain Behav Immun 56: 271-280. S0889-1591(16)30071-X [pii];10.1016/j.bbi.2016.03.026 [doi].
- Du S, Wu S, Feng X, Wang B, Xia S, Liang L, Zhang L, Govindarajalu G, Bunk A, Kadakia F, Mao Q, Guo X, Zhao H, Berkman T, Liu T, Li H, Stillman J, Bekker A, Davidson S, Tao YX (2022) A nerve injury-specific long noncoding RNA promotes neuropathic pain by increasing Ccl2 expression. J Clin Invest 132: e153563. 153563 [pii];10.1172/JCI153563 [doi].
- He L, Han G, Wu S, Du S, Zhang Y, Liu W, Jiang B, Zhang L, Xia S, Jia S, Hannaford S, Xu Y, Tao YX (2020) Toll-like receptor 7 contributes to neuropathic pain by activating NF-kappaB in primary sensory neurons. Brain Behav Immun 87: 840-851. S0889-1591(19)31555-7 [pii];10.1016/j.bbi.2020.03.019 [doi].
- Zhang L, Li X, Feng X, Berkman T, Ma R, Du S, Wu S, Huang C, Amponsah A, Bekker A, Tao YX (2022) E74-like factor 1 contributes to nerve trauma-induced nociceptive hypersensitivity via transcriptionally activating matrix metalloprotein-9 in dorsal root ganglion neurons. Pain . 10.1097/j.pain.0000000000002673 [doi];00006396-990000000-00080 [pii].
- Zhang Z, Zheng B, Du S, Han G, Zhao H, Wu S, Jia S, Bachmann T, Bekker A, Tao YX (2020) Eukaryotic initiation factor 4 gamma 2 contributes to neuropathic pain through downregulation of Kv1.2 and the mu opioid receptor in mouse primary sensory neurones. Br J Anaesth . S0007-0912(20)30911-9 [pii];10.1016/j.bja.2020.10.032 [doi].
- Wang PK, Cao J, Wang H, Liang L, Zhang J, Lutz BM, Shieh KR, Bekker A, Tao YX (2015) Short-Term Sleep Disturbance-Induced Stress Does not Affect Basal Pain Perception, but Does Delay Postsurgical Pain Recovery. J Pain 16: 1186-1199. S1526-5900(15)00809-3 [pii];10.1016/j.jpain.2015.07.006 [doi].
- Xu JT, Zhou X, Zhao X, Ligons D, Tiwari V, Lee CY, Atianjoh FE, Liang L, Zang W, Njoku D, Raja SN, Yaster M, Tao YX (2014) Opioid receptor-triggered spinal mTORC1 activation contributes to morphine tolerance and hyperalgesia. J Clin Invest 124: 592-603. S0006-8993(10)00818-8 [pii];10.1016/j.brainres.2010.04.010 [doi].
- Zhang J, Liang L, Miao X, Wu S, Cao J, Tao B, Mao Q, Mo K, Xiong M, Lutz BM, Bekker A, Tao YX (2016) Contribution of the Suppressor of Variegation 3-9 Homolog 1 in Dorsal Root Ganglia and Spinal Cord Dorsal Horn to Nerve Injury-induced Nociceptive Hypersensitivity. Anesthesiology 125: 765-778. 10.1097/ALN.0000000000001261 [doi].
- Miao XR, Fan LC, Wu S, Mao Q, Li Z, Lutz B, Xu JT, Lu Z, Tao YX (2017) DNMT3a contributes to the development and maintenance of bone cancer pain by silencing Kv1.2 expression in spinal cord dorsal horn. Mol Pain 13: 1744806917740681. 10.1177/1744806917740681 [doi].
- Xu JT, Sun L, Lutz BM, Bekker A, Tao YX (2015) Intrathecal rapamycin attenuates morphine-induced analgesic tolerance and hyperalgesia in rats with neuropathic pain. Transl Perioper Pain Med 2: 27-34.
- Yuan J, Wen J, Wu S, Mao Y, Mo K, Li Z, Su S, Gu H, Ai Y, Bekker A, Zhang W, Tao YX (2019) Contribution of dorsal root ganglion octamer transcription factor 1 to neuropathic pain after peripheral nerve injury. Pain 160: 375-384. 10.1097/j.pain.0000000000001405 [doi].
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53: 55-63. 0165-0270(94)90144-9 [pii].
- Dixon WJ (1980) Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 20: 441-462. 10.1146/annurev.pa.20.040180.002301 [doi].
- Cao J, Wang PK, Tiwari V, Liang L, Lutz BM, Shieh KR, Zang WD, Kaufman AG, Bekker A, Gao XQ, Tao YX (2015) Short-term pre- and post-operative stress prolongs incision-induced pain hypersensitivity without changing basal pain perception. Mol Pain 11: 73. 10.1186/s12990-015-0077-3 [doi];10.1186/s12990-015-0077-3 [pii].
- Chu YC, Guan Y, Skinner J, Raja SN, Johns RA, Tao YX (2005) Effect of genetic knockout or pharmacologic inhibition of neuronal nitric oxide synthase on complete Freund's adjuvant-induced persistent pain. Pain 119: 113-123. S0304-3959(05)00480-X [pii];10.1016/j.pain.2005.09.024 [doi].
- Fan L, Guan X, Wang W, Zhao JY, Zhang H, Tiwari V, Hoffman PN, Li M, Tao YX (2014) Impaired neuropathic pain and preserved acute pain in rats overexpressing voltage-gated potassium channel subunit Kv1.2 in primary afferent neurons. Mol Pain 10: 8. 1744-8069-10-8 [pii];10.1186/1744-8069-10-8 [doi].
- Tao YX, Rumbaugh G, Wang GD, Petralia RS, Zhao C, Kauer FW, Tao F, Zhuo M, Wenthold RJ, Raja SN, Huganir RL, Bredt DS, Johns RA (2003) Impaired NMDA receptor-mediated postsynaptic function and blunted NMDA receptor-dependent persistent pain in mice lacking postsynaptic density-93 protein. J Neurosci 23: 6703-6712. 23/17/6703 [pii].
- Tao YX (2012) AMPA receptor trafficking in inflammation-induced dorsal horn central sensitization. Neurosci Bull 28: 111-120. 10.1007/s12264-012-1204-z [doi].
- Huck NA, Siliezar-Doyle J, Haight ES, Ishida R, Forman TE, Wu S, Shen H, Takemura Y, Clark JD, Tawfik VL (2021) Temporal Contribution of Myeloid-Lineage TLR4 to the Transition to Chronic Pain: A Focus on Sex Differences. J Neurosci 41: 4349-4365. JNEUROSCI.1940-20.2021 [pii];10.1523/JNEUROSCI.1940-20.2021 [doi].
- Woller SA, Corr M, Yaksh TL (2015) Differences in cisplatin-induced mechanical allodynia in male and female mice. Eur J Pain 19: 1476-1485. 10.1002/ejp.679 [doi].
- Szabo-Pardi TA, Barron LR, Lenert ME, Burton MD (2021) Sensory Neuron TLR4 mediates the development of nerve-injury induced mechanical hypersensitivity in female mice. Brain Behav Immun 97: 42-60. S0889-1591(21)00242-7 [pii];10.1016/j.bbi.2021.06.011 [doi].