Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/176
Case Report | Volume 10 | Issue 2 Open Access
Successful Anesthetic Management of Pediatric Living Donor Liver Transplantation with Hepatopulmonary Syndrome and Pulmonary Arteriovenous Fistula due to Biliary Atresia: A Case Report
Çağla Yazar, MD*, Nedim Çekmen, MD, Adnan Torgay, MD
Department of Anesthesiology and Intensive Care Unit, Faculty of Medicine, Baskent University, Ankara, Turkey
Çağla Yazar, MD, Department of Anesthesiology and Intensive Care Unit, Faculty of Medicine, Baskent University, Fevzi Cakmak Caddesi 10, Sokak No: 45 Bahcelievler, 06490 Ankara, Turkey, Tel: 0312203-68-68-4813; +905454160779, E-mail: cglyzr@gmail.comEditor: Renyu Liu, MD; PhD; Professor, Department of Anesthesiology and Critical Care, Perelman School of Medicine at the University of Pennsylvania, Center of Penn Global Health Scholar, 336 John Morgan building, 3620 Hamilton Walk, Philadelphia, PA 19104, USA, Fax: 2153495078, E-mail: RenYu.Liu@pennmedicine.upenn.edu
Received: May 18, 2023 | Accepted: June 20, 2023 | Published: June 21, 2023
Citation: Yazar C, Çekmen N, Torgay A. Successful Anesthetic Management of Pediatric Living Donor Liver Transplantation with Hepatopulmonary Syndrome and Pulmonary Arteriovenous Fistula due to Biliary Atresia: A Case Report. Transl Perioper Pain Med 2023; 10(2):533-537
Abstract
Hepatopulmonary syndrome (HPS) is a severe, progressive, worsening complication of end-stage liver disease (ESLD). The primary triad of HPS is hypoxemia, intrapulmonary vascular dilatation (IPVD) and hepatic cirrhosis. Liver transplantation (LT) is an important treatment option for HPS, but it has high mortality and morbidity. Our patient, a 6-year-old girl, underwent a porto-enterostomy (Kasai) operation on the 45 th day of delivery due to biliary atresia. We present a 6-year-old female patient with successful anesthetic management of pediatric living donor LT with HPS and pulmonary arteriovenous fistula (AVF) due to biliary atresia. Anesthesiologists should know the comprehensive preoperative assessment and underlying pathophysiologic condition, determine an anesthesia plan for patients with HPS, meticulously account for HPS-related hypoxia and PVR conditions, and follow up closely perioperatively for successful outcomes.
Keywords
Biliary, Atresia, Hepatopulmonary syndrome, Pulmonary arteriovenous fistula, Anesthetic management
Introduction
A disturbance in arterial oxygenation characterizes HPS due to IPVD in ESLD (usually cirrhosis with portal hypertension). The three main manifestations of HPS are characterized by hepatic cirrhosis, IPVD, and an increased alveolar-arterial oxygen gradient (≥ 15 mmHg or ≥ 20 mmHg if age > 64) while breathing room air at rest in the sitting position [1,2]. HPS occurs in 4-47% of adults with ESLD [2], with an estimated prevalence of 3-20% in children HPS can occur [3]. HPS may lead to progressive dyspnea, platipne, orthodeoxia, cyanosis, and hypoxia, thus requiring supplemental oxygen on resting and/or exertion. The most definitive detection of IPVD is with contrast-enhanced transthoracic echocardiography (CE-TTE) [1,2]. Pharmacological trials have been performed to treat HPS but have not been sufficiently compelling. LT was contraindicated in previous years in ESLD patients with HPS due to potential preoperative and postoperative complications. However, in recent years, LT has been accepted as the only effective treatment in these patients with HPS. However, the risk of developing severe complications, mortality and morbidity after LT remains high, especially in severe HPS. In addition, in pulmonary AVF, typically in ESLD patients with HPS, the risk of development is very high [2,4]. Any change in PVR may affect and trigger the development of hypoxemia in HPS patients with or without AVF; therefore, these triggering factors must be avoided [1,2].
Herein, we present the successful anesthetic management of a pediatric living donor LT for biliary atresia with a pulmonary AVF and HPS in the light literature.
Case Report
A 6-year-old female (height: 115 cm, weight: 22 kg) patient with pediatric living donor LT for biliary atresia (cirrhosis with portal hypertension) with a pulmonary AVF, HPS, was admitted to an intensive care unit (ICU) transplantation. She had a Kasai operation on the 45 th day after birth to treat biliary atresia. After the Kasai procedure, the patient developed malnutrition due to portal hypertension, hepatosplenomegaly, jaundice, ascites, and esophageal varices. After month 5, dyspnea, platypnea, orthodeoxia, weakness, cyanosis when crying, clubbing, and cyanotic fingertips began to appear. These findings' presence in ESLD patients made us think of HPS, and CE-TTE was then performed, and microbubbles were observed in the left atrium on CE-TTE. A lung perfusion scan revealed an intrapulmonary right-to-left shunt fraction of 25%, and pulmonary arteriography was also diffuse IPVD and pulmonary AVF without capillary phase was detected. In the patient's liver computed tomography (CT)-angiography, diffuse hepatoportal shunt and splenomegaly were found in the liver parenchyma due to the operated biliary atresia (Figure 1). She later developed cirrhosis and was followed up with a diagnosis of cirrhosis until the age of 6 years.
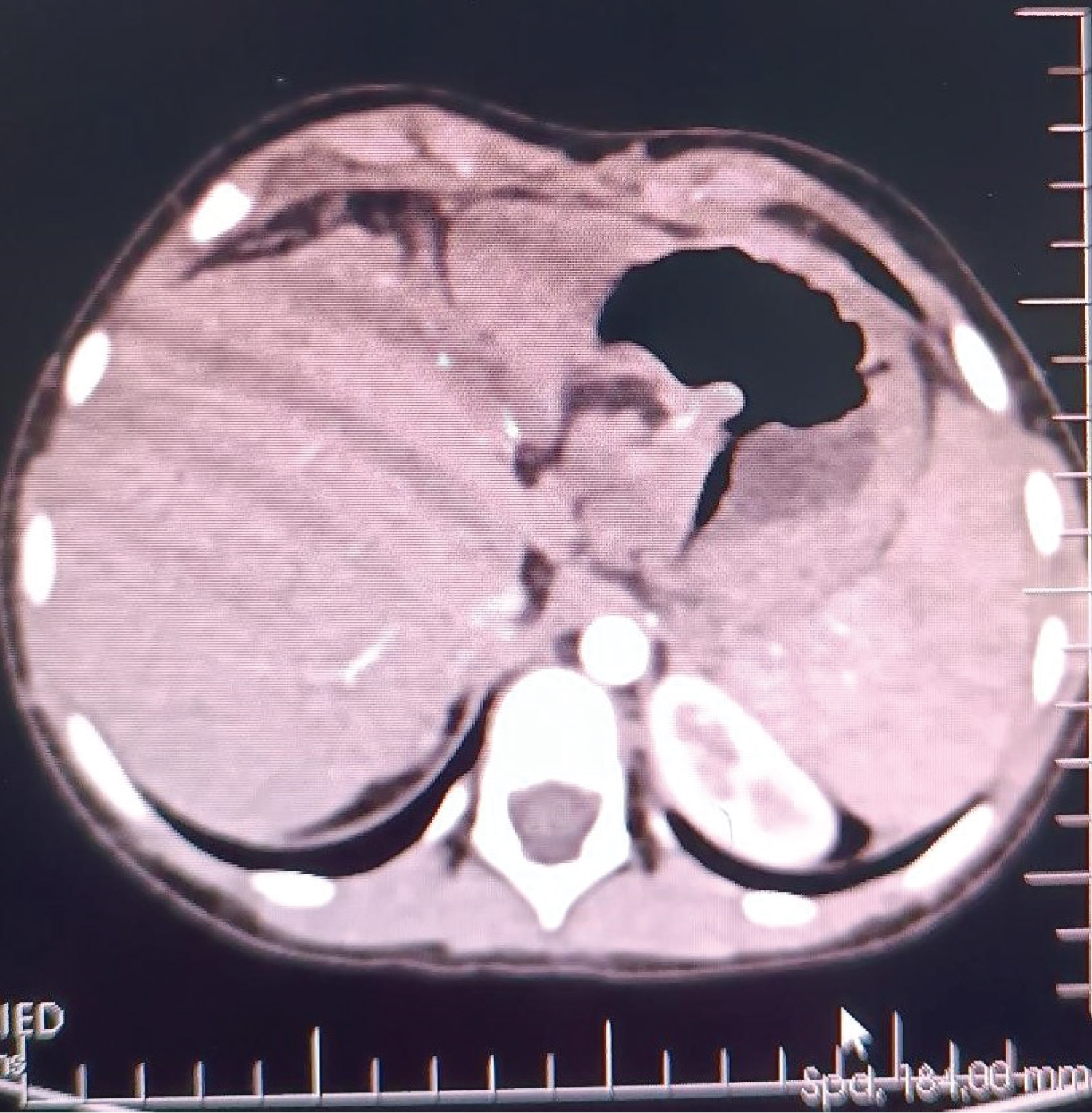
Figure 1: In the patient's liver computed tomography (CT) - angiography, diffuse hepatoportal shunt and splenomegaly were found in the liver parenchyma due to the operated biliary atresia.
Over time, a living donor LT is planned due to the patient's decreased arterial oxygen saturation (SaO 2 = 85%), dyspnea, platypnea, orthodeoxia, worsened cyanosis, and hypoxemia. Preoperative pulmonary function tests and chest X-rays were normal, electrocardiogram (ECG) revealed a regular sinus rhythm. Pulmonary hypertension was not detected in TTE. The venous blood analysis performed in the supine and sitting positions in room air determined orthodeoxia. While venous oxygen pressure and O 2 saturation were 56.5 mmHg and 86.3% in the supine position, it was 43.5 mmHg and 69.8% in the sitting position. Preoperative laboratory values: hemoglobin, 13.2 g/dL; hematocrit, 39.6%; platelet, 35 × 103/mL; aspartate transaminase level, 97 IU/L; alanine aminotransferase level, 115 IU/L; gamma-glutamyl transferase, 67 IU/L; total bilirubin, 1.8 mg/dL, direct bilirubin, 0.6 mg/dL, alkaline phosphatase level, 228 IU/L; albumin, 3.1 g/dL, international normalized ratio (INR) 1.26; prothrombin time (PT), 13.3 s; blood urea nitrogen (BUN) and creatinine values were normal limits.
The Child-Pugh-Turcotte (CPT) score was B, and the pediatric end-stage liver disease (PELD) score was 6.7. We obtained written and verbal informed consent from the patient's parents to publish our case. The patient was taken to the operating room without premedication. Pulse oximetry (SpO 2 ) was 84-88% with room air. The patient's American Society of Anesthesiologists (ASA) physical classification was class III, and Mallampati scored II. The living donor of the patient was her father, who was 35-years-old, and her ASA I and Mallampati scores were II. The patient was prepared for removal of the left lateral lobe.
Standard monitoring was applied to the patient, including ECG, SpO 2 , noninvasive blood pressure, capnography, temperature and bispectral index. After preoxygenation with 80% O 2 for 3 minutes, induction was performed with lidocaine 1 mg/kg, propofol 2-3 mg/kg, fentanyl 1 μg/kg and rocuronium 0.6 mg/kg and with a number 4.5 cuffed endotracheal tube without difficulty. The patient was intubated without complication and was connected to the ventilator. The patient's ventilator parameters were adjusted appropriately. After induction, a left radial artery catheter for hemodynamic monitoring and a left femoral artery catheter for PiCCO monitoring and a right subclavian central venous catheter was placed. Anesthesia was maintained with 0.01-0.05 μg/kg/min remifentanil infusion, 0.3 mg/kg/h rocuronium infusion, 2% sevoflurane and 50% oxygen + 50% air mixture. In the intraoperative period, norepinephrine infusion (0.01-0.15 mg/kg/min) was started for hypotension.
We aimed for mean arterial pressure (MAP) > 60 mmHg throughout the operation. 560g of liver tissue was removed from the patient (Figure 2). After the anastomoses of the great vessels were completed in the anhepatic phase of approximately 45 minutes, the clamp on the portal vein was removed, and reperfusion was achieved. The patient's blood pressure fell to 66/34 mmHg 2 minutes after reperfusion. The norepinephrine infusion dose was increased until his hemodynamics stabilized, and 5 mg of ephedrine and 100 mg of calcium chloride were given twice. In the intraoperative period, transesophageal echocardiography (TEE) provides invaluable information for closely monitoring pulmonary vascular resistance (PVR) and right ventricular functions in these patients. Since our patient had esophageal varices, we did not perform direct TEE, but when an extraordinary event occurred, we kept her at the patient's bedside to do it quickly. Our patient did not have pulmonary hypertension in the preoperative examination, and we tried to minimize the triggering factors that would increase PVR throughout the operation. We did not detect any increase in PVR in the measurement we made with PiCCO during the neo-hepatic phase. In the neo-hepatic phase, the dose of norepinephrine was reduced as the patient's hemodynamics returned to normal. Fluid therapy was performed considering the PiCCO parameters. The estimated blood loss during surgery was less than 250 mL. During the surgery, the patient was given a total of 1300 mL crystalloid, 1300 mL 5% albumin-containing colloid and 200 mL cell-caver, and 450 mL urine output was achieved. The inserted liver weighed 285g (Figure 3), and the operation continued for 7 hours. After the operation, she was taken to the intubated ICU and connected to a mechanical ventilator. While the patient was being followed on the ventilator, her SaO 2 was between 0.46 and 86-92%. On the 2 nd postoperative day, SaO 2 remained at 90-95%, hypoxemia resolved, weaning was applied, and the patient was weaned from the ventilator. After 2 days of oxygen support with a 2 L/min mask, she breathed in normal room air. After extubation, the patient's general condition and neurological examination were normal. Blood gas values, lactate level and hepatic transaminases returned to normal within days. Immunosuppressive drugs, steroids and nutritional support were administered to the patient. The patient was transferred to the service on the 4 th day after her follow-up in the ICU, and her follow-up continued. The patient was discharged 15 days after the operation without complications in the postoperative period.
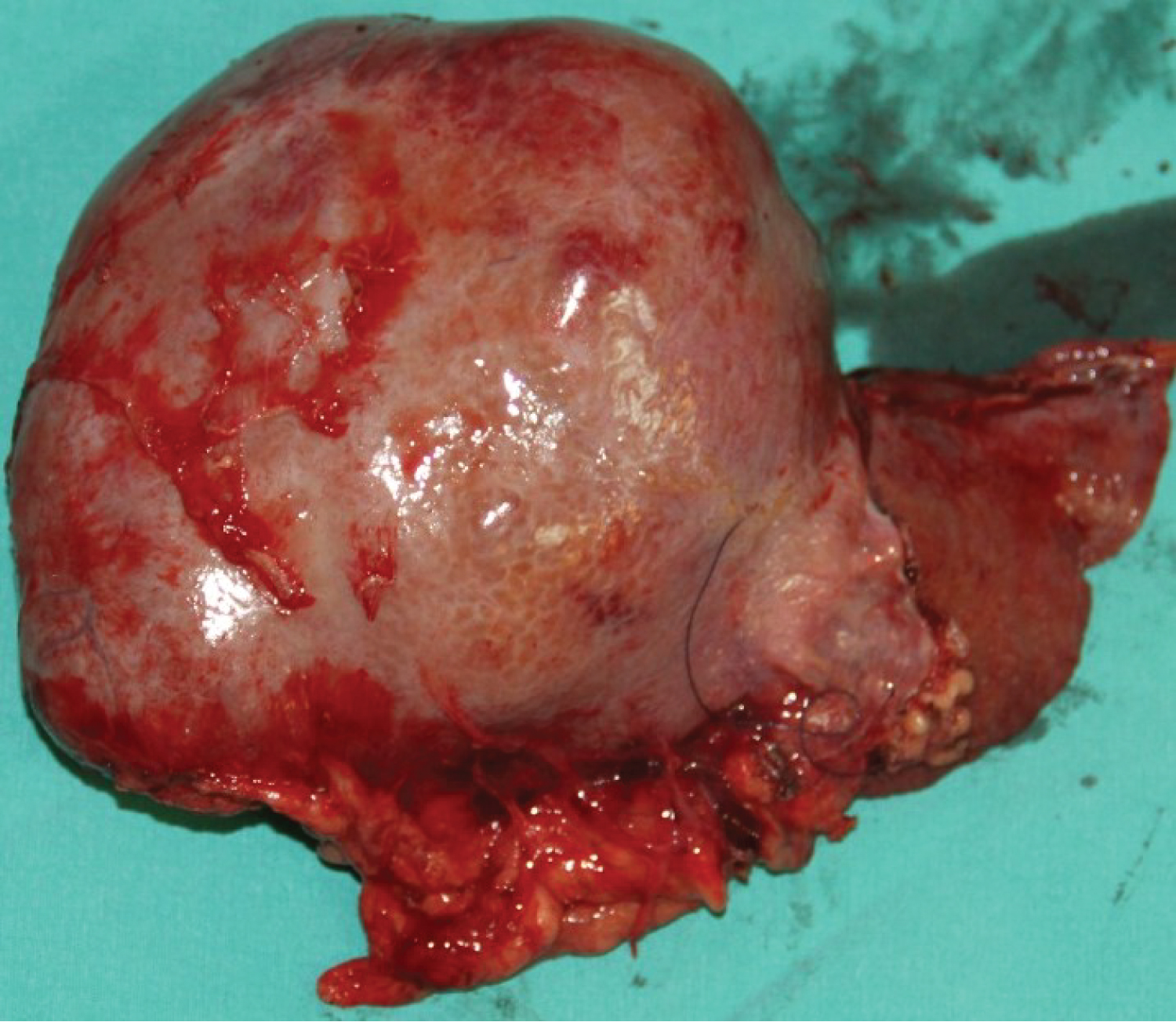
Figure 2: 560g of liver tissue was removed from the patient.
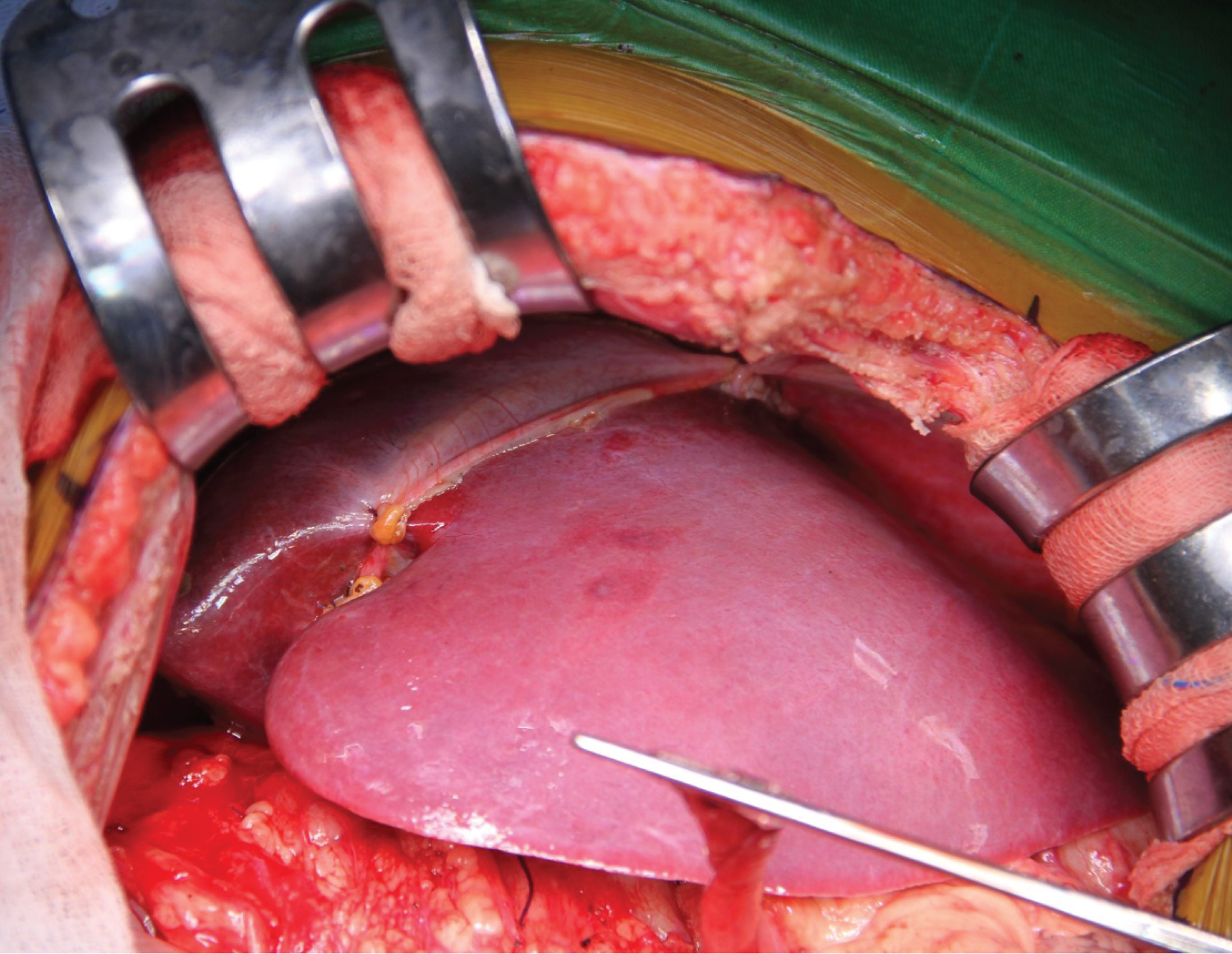
Figure 3: The inserted liver weighed 285g.
Discussion
HPS is an essential pulmonary vascular complication seen in patients with ESLD. The most common clinical findings of HPS include hepatosplenomegaly, jaundice, ascites, and cutaneous spider nevi associated with ESLD, as well as concurrent exertional dyspnea, platypnea, orthodeoxia, clubbing, and apical cyanosis [1,2]. The mortality rate of HPS in children without LT is up to 48%. Respiratory, multi-organ failure, infection, pulmonary and intracranial hemorrhage, and portal vein thrombosis are among the most important causes of death [3,4].
HPS was previously known as a contraindication to LT, mainly in patients with severe hypoxemia [3]. The mortality rate in LT candidates with a PaO 2 of less than 50 mmHg in room air preoperatively was 30% reported. However, it should be kept in mind that mechanical ventilators may be needed for a long time, even after successful LT [3,4]. Venous oxygen pressure and O 2 saturation were 56.5 mmHg and 86.3% versus 43.5 mmHg and 69.8% for each position measured in our patient in the preoperative period for detecting orthodeoxia. We successfully performed LT in a 6-year-old girl with HPS, pulmonary AVF (SpO 2 , with room air, was 84-88%), intrapulmonary right-to-left shunt fraction of 25%, and diffuse IPVD with ESLD due to biliary atresia.
Patients with HPS may present with abnormally enlarged alveolar capillaries, which may cause intrapulmonary right-to-left shunting and, in severe cases, the development of a pulmonary AVF [1,3]. Hypoxemia may aggravate the clinical picture in these patients with pathological pulmonary AVF in HPS. With or without pulmonary AVF, reductions in PVR and conditions that increase pulmonary vasodilation, which can worsen hypoxemia, must be avoided to administer anesthesia to patients with HPS safely. Therefore, PVR is critical in these patients, and hypoxic pulmonary vasoconstriction (HPV) is crucial in sustaining PVR under normal conditions [4,5].
Inherent patient factors influencing HPV involve pulmonary arterial pressure (PAP), PVR, mixed venous oxygen partial pressure (PvO 2 ), and partial carbondioxide pressure (PaCO 2 ). These factors can be directly controlled by anesthesiologists using vasodilators, inhalation anesthetics, positive end‐expiratory pressure (PEEP), and nitric oxide (NO) [5,6]. We tried to take precautions as carefully as possible by considering these factors in our anesthesia plan. We did not prefer inhalation anesthetics (Sevoflurane) as they may adversely affect HPV, and we used propofol and remifentanil for the maintenance of anesthesia since we know that propofol and remifentanil do not adversely affect HPV. Additionally, we applied PEEP between 4-6 mmHg and tried to keep PaCO 2 within the normal range.
A different pulmonary AVF distinguished our case from cases where the intrapulmonary shunt was due solely to pulmonary vasodilation. HPV response is weaker than normal in patients with ESLD because of impaired and decreased pulmonary vascular tone. Such as phenylephrine and ephedrine to protect against HPV in patients with AVF and shunts vasoconstrictor drugs may be required. However, they should be avoided as much as possible as they can increase the shunt. We also prepared drugs such as NO should be available in these patients to prevent hypoxemia [5,6]. Low-dose NO has been found to reduce hypoxia in the postoperative period in patients with HPS [6]. Hypoxemia did not develop in our case, and we didn't need NO. Methylene blue is a potent NO inhibitor and may therefore contribute to treating hypoxemia caused by pulmonary vasodilation in HPS patients. However, methylene blue significantly increases the vasoconstrictor effect. In patients with pulmonary AVF, it increases intrapulmonary shunt; therefore, administration should be avoided [7]. Since our case had HPS and AVF, we closely applied PiCCO to monitor the difficulties of possible hemodynamic and oxygenation changes. These patients should be continuously monitored with PiCCO to identify any imbalances between oxygen delivery and consumption. This type of follow-up maybe it helps identify any causative factors and provides convenience for quick patient management [2].
PRS is the reduction in SVR and blood pressure, an increase in PAP characterizes after portal clamp release during LT, and PRS may deteriorate hypoxia by raising the shunt fraction in patients with pulmonary AVF and HPS [6]. PRS occurred in our case but did not significantly impair the patient's hemodynamics, hypoxemia did not develop, and everything returned to normal after about 2 minutes. These HPS and pulmonary AVF patients are at high risk for paradoxical embolism. For this reason, air entry into the surgical area should be prevented during the operation, and the formation of air bubbles should be prevented when fluid or medication is given, we paid attention to these. Although PEEP can prevent paradoxical embolism, it is not recommended due to the potential for increased PVR [4-6]. We tried to keep the peep as low as 4-6 mmHg.
Park TJ, et al. [8] reported that on a 16-year-old patient with HPS CPT classification C and MELD score of 30 in severe ESLD with intrapulmonary arterio-venous shunt findings and hypoxemia detected by CE-TTE, performed LT, and the patient recovered entirely after LT. In our case, we suspected HPS because of these ESLD-related findings in our patient, and then CE-TTE was performed. Microbubbles in the left atrium in CE-TTE and intrapulmonary right-to-left shunt fraction were 25% in lung perfusion scan, and diffuse IPVD in pulmonary arteriography was detected.
Lee HJ, et al. [9] reported that for a 9-year-old girl with ESLD due to biliary atresia and HPS and diffuse pulmonary AVF living donor LT was performed on this patient. They applied the total intravenous anesthesia (TIVA) method as anesthesia management to protect HPV and prevent shunt, hypoxemia and PVR increase, and they successfully performed the LT. We successfully performed LT in a 6-year-old girl (Child class B, PELD score is 6.7) with HPS, pulmonary AVF, intrapulmonary right-to-left shunt fraction of 25%, and diffuse IPVD with ESLD due to biliary atresia. In our anesthesia plan, we tried to avoid factors that would trigger shunt, hypoxemia and PVR increase, and we used propofol and remifentanil for anesthesia maintenance. We have NO available to treat possible hypoxemia attacks due to HPS in the intraoperative period.
Recovery time of lower PaO 2 and intrapulmonary shunt after LT are variable, depending on the severity of preoperative lower PaO 2 . Although lower preoperative PaO 2 is related to a more extended recovery period of HPS resolution after LT, in most cases, HPS can be resolved within 1-year [2]. In a study by Kianifar HR, et al. [10], pediatric patients exhibited significant increases in PaO 2 , SaO 2 , decreased alveolar-arterial oxygen gradient, and pulmonary AV fistula following 3-6 months of treatment with pediatric LT. While our patient was being followed on the ventilator, her SaO 2 was between 0.46 and 86-92%. On the 2 nd postoperative day, SaO 2 remained at 90-95%, hypoxemia resolved, weaning was applied, and the patient was weaned from the ventilator. After 2 days of oxygen support with a 2 L/min mask, she breathed in normal room air.
Conclusion
Early detection of HPS and detection of possible risk factors in patients with ESLD are critical. In patients with HPS and pulmonary AVF, LT is the only treatment, and successful LT can lead to complete regression of the shunt, improved oxygenation, and improved quality of life. It is essential to protect HPV and reduce sudden changes in PVR to prevent adverse effects that may cause hypoxia in the anesthesia management of these patients who will undergo LT. Anesthesiologists should know the comprehensive preoperative assessment and underlying pathophysiologic condition, determine an anesthesia plan for patients with HPS, meticulously account for HPS-related hypoxia and PVR conditions, and follow up closely perioperatively for successful outcomes.
Conflict of Interest
None.
Funding
No funding.
Acknowledgment
None.
Ethical Statement
Başkent University Hospital human research ethics committee approval was attended for writing this case report.
References
- Rodriguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome- a liver-induced lung vascular disorder. N. Engl. J. Med. 2008; 358: 2378-2387.
- Krowka MJ, Fallon MB, Kawut SM, et al. International liver transplant society practice guidelines: Diagnosis and management of hepatopulmonary syndrome and portopulmonary hypertension. Transplantation. 2016; 100: 1440-1452.
- Warner S, McKiernan PJ, Hartley J, et al. Hepatopulmonary syndrome in children: A 20-year review of presenting symptoms, clinical progression, and transplant outcome. Liver Transplant. 2018;24(9): 1271-1279.
- Younis I, Sarwar S, Butt Z,Tanveer S, Qaadir A, Jadoon NA. Clinical characteristics, predictors, and survival among patients with hepatopulmonary syndrome. Ann Hepatol. 2015; 14: 354.
- Lumb AB, Slinger P. Hypoxic pulmonary vasoconstriction. Physiology and anaesthetic implications. Anesthesiology. 2015; 122: 932-946.
- Rodriguez-Roisin R, Roca J, Agusti Ag, Mastai R, Wagner Pd, Bosch J. Gas exchange and pulmonary vascular reactivity in patients with liver cirrhosis. Am Rev Respir Dis. 1987; 135: 1085-1092.
- Koelzow H, GedneyJa, Baumann J, Snook Nj, Bellamy Mc. The effect of methylene blue on the hemodynamic changes during ischemia-reperfusion injury in orthotopic liver transplantation. Anesth Analg. 2002: 94: 824-829.
- Park TJ, Ahn KS, Kim YH, et al. Improved severe hepatopulmonary syndrome after liver transplantation in an adolescent with end-stage liver disease secondary to biliary atresia. Clin Mol Hepatol. 2014;20: 76-80.
- Lee HJ, Lee JM, Jung CW, Lee J. Anesthetic management of a pediatric patient with pulmonary arteriovenous fistula undergoing liver transplantation: A case report. Pediatr Transplant.2016; 20: 711-716.
- Kianifar HR, Khalesi M, Mahmoodi E, Afzal Aghaei M. Pentoxifylline in hepatopulmonary syndrome. World J Gastroenterol. 2012;18(35): 4912-4916.