Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/189
Review Article | Volume 11 | Issue 2 Open Access
Current Perioperative Management of CSF Drains for Repair of TAAA and DTAA Surgery
Shao Feng Zhou, MD2, Akiko Tanaka, MD, PhD2 and Anthony Estrera, MD2
1Department of Cardiothoracic and Vascular Anesthesiology, McGovern Medical School at UTHealth Houston, Houston, Texas, USA
2Department of Cardiothoracic and Vascular Surgery, McGovern Medical School at UTHealth Houston, Houston, Texas, USA
Shao Feng Zhou, MD, Professor, Department of Anesthesiology, Critical Care and Pain Medicine, McGovern Medical School at UTHealth Houston, 6431 Fannin St., MSB. 5.020, Houston, TX, 77030, USA, Tel: +1-713-500-6201, E-mail: shao.feng.zhou@uth.tmc.eduEditor: Renyu Liu, MD; PhD; Professor, Department of Anesthesiology and Critical Care, Perelman School of Medicine at the University of Pennsylvania, Center of Penn Global Health Scholar, 336 John Morgan building, 3620 Hamilton Walk, Philadelphia, PA 19104, USA, Fax: 2153495078, E-mail: RenYu.Liu@pennmedicine.upenn.edu
Received: August 21, 2024 | Accepted: September 26, 2024 | Published: September 28, 2024
Citation: Zhou SF, Tanaka A, Estrera A. Current Perioperative Management of CSF Drains for Repair of TAAA and DTAA Surgery. Transl Perioper Pain Med 2024; 11(2):620-630
Abstract
As both open and endovascular surgery for descending thoracic aortic aneurysms (DTAA) and thoracoabdominal aortic aneurysm (TAAA) have increased, there has also been an increase in the request for cerebrospinal fluid drainage (CSFd). Routinely performed in elective TAAA, a lower lumbar approach drain catheter is placed preoperatively and CSF is intermittently drained with the goal of maintaining intracranial pressure (ICP) less than central venous pressure (CVP) or less than 10 mmHg. This adjunct technique is continued into the postoperative period until the patient has maintained intact neurologic function. Most CSFd placement can be elective.
Once the CSFd catheter is placed, CSFd management starts its individual labor consumption. It must continuously deal with CSFd based on the patient’s medical and neurologic condition. Troubleshooting is frequently required for problems associated with CSFd, as perioperative management can be quite challenging for the entire aortic team. Currently, there are no standard protocols for perioperative management of CSFd in TAAA and DTAA patients. Its handling is dependent on the rapid change of patient condition and clinical performance, the risk from diseases itself, and the complexity of the surgery.
This paper introduces management CSFd and its associated complications from our aortic center, which has more than 25 years of clinical experience. Our aim is to emphasize the performance and management CSFd by using neuromonitoring-guided surgical condition as well as patient clinical condition. We are first to suggest the management of prophylactic CSFd and therapeutic CSFd intraoperatively in TAAA and DTAA surgeries. Further studies are needed using neuromonitoring-guided CSFd in these repairs.
Keywords
Cerebrospinal fluid drainage, Thoracoabdominal aortic aneurysm, TAAA, Spinal cord protection, Intraoperatively neuromonitoring, SSEP, MEP
Abbreviations
CSFd: Cerebrospinal Fluid drainage; CSFP: Cerebrospinal Fluid Pressure; CVP: Central Venous Pressure; DTAA: Descending Thoracic Aortic Aneurysms; ICH: Intracranial Hemorrhage; ICP: Intracranial Pressure; ICU: Intensive Care Unit; MAP: Mean Aortic Pressure; MEP: Motor Evoked Potentials; SCI: Spinal Cord Injury; SCPP: Spinal Cord Perfusion Pressure; SSEP: Somatosensory Evoked Potentials; SvO 2 : venous Oxygen Saturation; TAA: Thoracic Aortic Aneurysms; TAAA: Thoracoabdominal Aortic Aneurysm
Background
The pooled incidence of thoracic aortic aneurysms (TAA) is 5.3 per 100,000 individuals/year and ruptured TAA was 1.6 per 100,000 individuals/year [1]. With the continuous development of imaging technology, advancement in the identification and diagnosis of thoracic aortic disease increased by 52% in men and 28% in women, reported in a nationwide, population-based study of more than 14,000 cases from 1987-2002 [2].
As both surgical and endovascular techniques have improved, open, endovascular, and hybrid repairs of TAAAs have all been extensively described. Regardless of the operative approach, organ protection has always been addressed to reduce overall morbidity and mortality. CSFd is one of the well-established strategies to reduce ICP, prevent spinal cord ischemia perioperatively, and lower the risk of paraplegia, which is associated with a significant increase in both morbidity and mortality in DTAA and TAAA surgeries [3,4]. Multiple studies have reported the incidence of postoperative paraplegia is significantly reduced by the CSFd technique in TAAA and DTAA repair surgery. Effective monitoring and adjustment of spinal cord perfusion pressure (SCPP) with the use of a CSFd drain is a class I level of evidence, recommended by the American Association for Thoracic Surgery and American Heart Association Task Force on Practice Guidelines in the 2010 US Guidelines for Spinal Cord Protection During Thoracic Aortic Repair [5] and, if needed, in intercostal artery preimplantation [6]. Intraoperative neuromonitoring using somatosensory evoked potentials (SSEP) and motor evoked potentials (MEP) are not only useful during TAAA and DTAA repair but also guide the timing to start the amount of CSF drainage to achieve lower ICP. It is combined with other alterations strategies, such as maintaining adequate spinal cord perfusion pressure, blood pressure, and cardiac output, which are beneficial in increasing perfusion to the collateral network of the spinal cord. Increased red blood cell volume to increase oxygen carriers may also be considered.
Complications of CSFd are not common but can be severe and even lethal. The most common are post-dura puncture headaches (with and without CSF leakage), CSF leakage, and blood-tinged CSF vs. bloody CSF. (If intracranial hemorrhage [ICH] subarachnoid hemorrhage, meningitis accurse can be quite severe and even lethal [7]). However, there are several studies that suggest CSFd is an important component of spinal cord protective strategy with favorable neurologic outcomes. The significant benefit of CSFd in TAAA and DTAA can be applied safely with excellent technical success and low complication rates. From our clinical experience, it can be performed safely perioperatively by well-trained teams [8,9].
As DTAA cases and TAAA surgeries increase, there has also been an increase in the request for CSFd insertion of both open and interventional repaired surgery. Routinely in elective TAAA repair, a lower lumbar drain catheter is placed preoperatively and CSF is intermittently drained with the goal of maintaining ICP < 10 mmHg. This adjunct technique is continued into the postoperative period until the patient has maintained intact neurologic function. The CSFd placement can be elective in the operation with a time limit determined at bedside. Once CSFd has begun, catheter placement, CSFd management starts its individual labor consumption. Perioperative management of CSFd are quite challenging for the aortic team, which includes the surgeon, anesthesiologist, intensivists, and intensive care unit (ICU) nurses. This paper will discuss the management of CSFd and its associated complications from our aortic center with more than 25 years of clinical experience.
The theoretical basis of CSFd
The artery of Adamkiewicz has been widely recognized for its importance in descending aortic surgery. It supplies the anterior spinal artery along the lower thoracic, lumbar, and sacral segments of the spinal cord, especially the spinal cord of the lumbar enlargement. It typically arises from the left side of the aorta between T8 and L2 (usually T9 to T12, although the artery of Adamkiewicz is found above T8 in about 15% of people), and has been documented as having a diameter from 0.6-1.8 mm. The variants of artery of Adamkiewicz include arising from the right side of the aorta or level outside of T8 through L2, differences in the angle of how the artery joins the anterior spinal artery, and the presence of more than one artery of Adamkiewicz [10]. Injury or occlusion to this artery can result in devastating ischemia of the corresponding spinal cord blood supply.
The anterior spinal artery is only one component of an extensive paraspinous and intraspinal collateral vascular network, which provides an anatomic explanation of the physiological resiliency of spinal cord perfusion when segmental arteries are occluded during the surgery. There are four levels of blood supply: Subclavian arteries; intercostal arteries; lumbosacral arteries; and hypogastric arteries. The hypogastric arteries -recognized as an important source of blood supply to the spinal cord- provide a physiologic explanation for the clinical observation that perfusion of the distal aorta. Clinical implications of the collateral network concept are important in providing adequate blood flow, based on the collateral network critically to both intraoperative and postoperative care [11-13].
CSF is produced by the choroid plexus and walls of the ventricles at about the rate of 20 mL/h, which varies in a diurnal rhythm (12-40 ml/h), and about 400-to-600 mL of CSF are normally produced in a 24-hour timespan. The total circulation volume of intrathecal fluid is 140-165 ml, turning 3.7 times per day. CSF continuously circulates between the spinal canal and the brain and is reabsorbed by the venous sinuses. CSF production increases in response to ischemia, which brings up increased cerebral blood pressure after the proximal aorta is clamped. CSFd is an adjectival and irreplaceable resource to optimize spinal cord perfusion pressure. The rate of production and circulating volume of CSF impacts the volume and rate of CSF drainage. CSF drainage that is too fast may induce severe complications, such as ICH. When spinal artery occlusion occurs during surgery, spinal blood supply is dependent on the collateral network and lower ICP by CSF drainage.
Strategies for spinal cord protection
Current spinal cord protection strategies designed to avoid spinal cord injury (SCI) during DTAA and TAAA repair are based on the causes during surgical procedures. Intraoperatively minimizing the short spinal cord ischemic time from the aortic clamp, effective distal aortic perfusion, in combination with CSFd, and moderate hypothermia (32°-34 °C) has been found clinically effective [14]. Reimplantation of intercostal arteries remains the best way to reduce the incidence of SCI and the risk of delayed paraplegia. Intraoperative SSEP and MEP monitors can warn that segmental vessels may be a necessary replacement, since implantation itself may complicate the procedure and lengthen the period of unavoidable intraoperative ischemia [15].
Monitoring for spinal cord protection
Neurophysiologic monitoring (Figure 1) of MEP and SSEP should be used to provide real-time, sensitive, rapidly responsive assessment of spinal cord perfusion status during DTAA or TAAA repairs. The monitoring of spinal cord function could be the earliest time to recognize the causes of spinal cord hypoperfusion or ischemia during the surgery. It is critically important to minimize SCI, which is typically due to failure to provide an adequate blood supply to the spinal cord perioperatively. In addition, neurophysiologic monitoring provides information of the patient in the risk of development of potentially delayed paraplegia postoperatively. MEP and SSEP monitoring of spinal cord ischemic status has reduced the risk of neurologic complications. On the other hand, adjacent management by the anesthesiologist will be utilized to improve flow, such as increasing SCPP and CSFd.
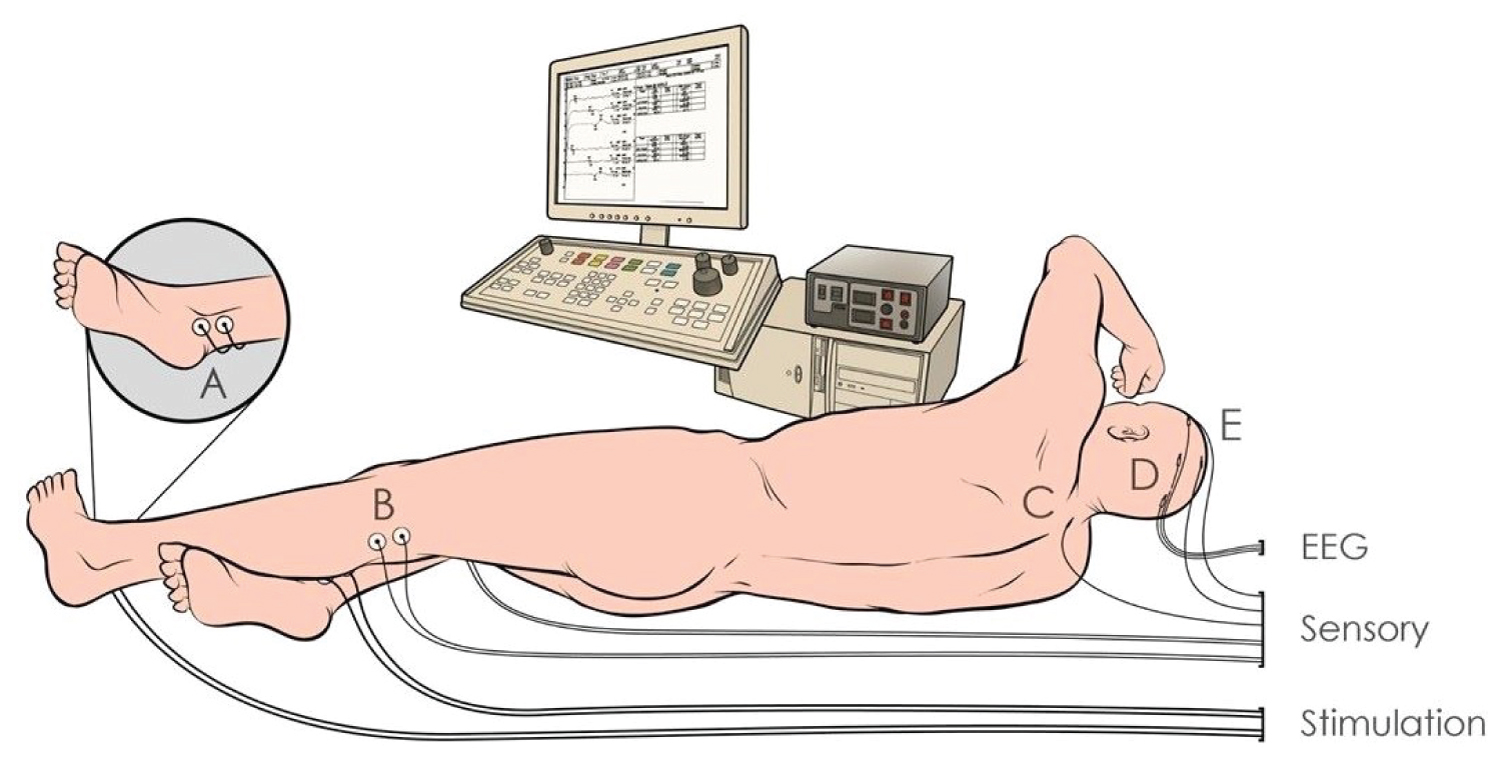
Figure 1:
Schematic diagram of neurophysiologic monitoring for DTAA and TAAA.
DTAA: Descending Thoracic Aortic Aneurysms; EEG; Electroencephalogram; MEP: Motor-Evoked Potentials; SSEP: Somatosensory-Evoked Potentials; TAAA: Thoracoabdominal Aortic Aneurysm.
There are significant hemodynamic changes during aortic cross-clamping that dynamically interact with CSF hydrodynamics and change in ICP. The placement of a spinal drain is based on reducing CSF pressure or ICP to optimize SCPP and maintain oxygen supply to the spinal cord.
CSFd has shown significant function in spinal cord protection and has been used by most centers intraoperatively and postoperatively to improve blood perfusion of the spinal cord. Simultaneously, hemodynamic parameters are important and necessary reference values to guide CSFd.
According to the formula, SCPP=MAP-(ICP or CVP [whichever is greater]), maximization and stability of hemodynamic parameters is mandatory perioperatively. Mean arterial pressures (proximal or distal) should be maintained at 80-100 mmHg and systolic pressure should be maintained at 130-150 mmHg to optimize spinal cord perfusion during the aortic clamp. Perfusion through the collateral network is undoubtedly enhanced by high-perfusion pressures if MEP monitoring demonstrates spinal cord ischemia during the clamp period [16].
It is also important to control CVP at a lower level, since increased venous congestion is destructive to spinal cord perfusion. Maintaining the patient’s baseline cardiac function adequate cardiac output and pulmonary artery pressure.
Venous oxygen saturation (SvO 2 ) is dependent on oxygen delivery and oxygen extraction. When the oxygen supply is insufficient to meet the metabolic demands of the tissues, an abnormal SvO 2 ensues and reflects an inadequacy in systemic oxygenation. With normal cardiac function, oxygen delivery can be maximized with the maintenance of normal hematocrit levels (oxygen carry ability) and careful attention to ventilation, so that PO 2 is normal. To maximize oxygen delivery to the spinal cord, it is important to avoid perioperative hypotension, anemia, hypocarbia/hypercarbia, and acidosis/alkalosis.
When and where the Csfd catheter should be placed
There is no uniform standard of when and where to place the CSFd. Some institutions place it immediately before the surgery in the ICU or interventional radiology unit so that any complications can be identified early to mitigate the likelihood of delayed surgery due to failed CSFd catheter placement. Other institutions perform it on awake patients, which allows for patient feedback of paresthesia or pain, which serve as neurological monitoring to minimize the potential nerve injury related to needle insertion or catheter placement. Our institution prefers placing CSFd in the operation room after general anesthesia after necessary lines up before surgery. It is beneficial for the patient to be comfortable as a lower level of anxiety reduces the time that the catheter stays in the subarachnoid space. It is cost-efficient that the catheter is placed on the same day of admission to the hospital. However, there is an increased risk of delayed surgery if there is bloody CSF drainage or failed CSF catheter insertion. Potential nerve injury related to needle insertion or catheter placement is very low if the insertion is below lumbar level 2 with a midline approach technique.
Placement of CSFd catheters
We use the INTEGRA External Drainage Catheters (INS 8330) with ventricular drainage systems (NL850-500V). There are questions of whether pre-CSFd catheter insertion needs prophylactic antibiotics. Our hospital policy is that prophylactic antibiotics are routinely administered with DTAA and TAAA surgeries 60 minutes prior to surgical incision.
Setup transducer for ICP is the same technique as setup for CVP. Primer transducer tube system with 0.9% normal saline, not heparin-contained solution, is used. The zeroing transducer position is at the level of the right atrium lever with supine and the lateral decubitus position intraoperatively. At this position, the head and lumber spinal cord is at the same level so that the ICP is equated to lumber cerebrospinal fluid pressure (CSFP). In this position, zeroing transducer position should be at the level of the CSFd catheter insertion point for acutely measured lumber CSFP.
The lateral decubitus with fetal position is better in the lumbar flexion to open intervertebral space (Figure 2), lower hydrostatic pressure of the column of CSF compared to the sitting position, minimizing the unexpected amount of CSF leaking from the 14G Tuohy stainless steel needles after puncturing the dura and during insert catheter. The midline approach is preferred instead of the paramedian line approach to avoid injury of epidural venous plexus and soft tissue injury. However, regardless of position, there is always a potential risk of unexpected venous puncture.
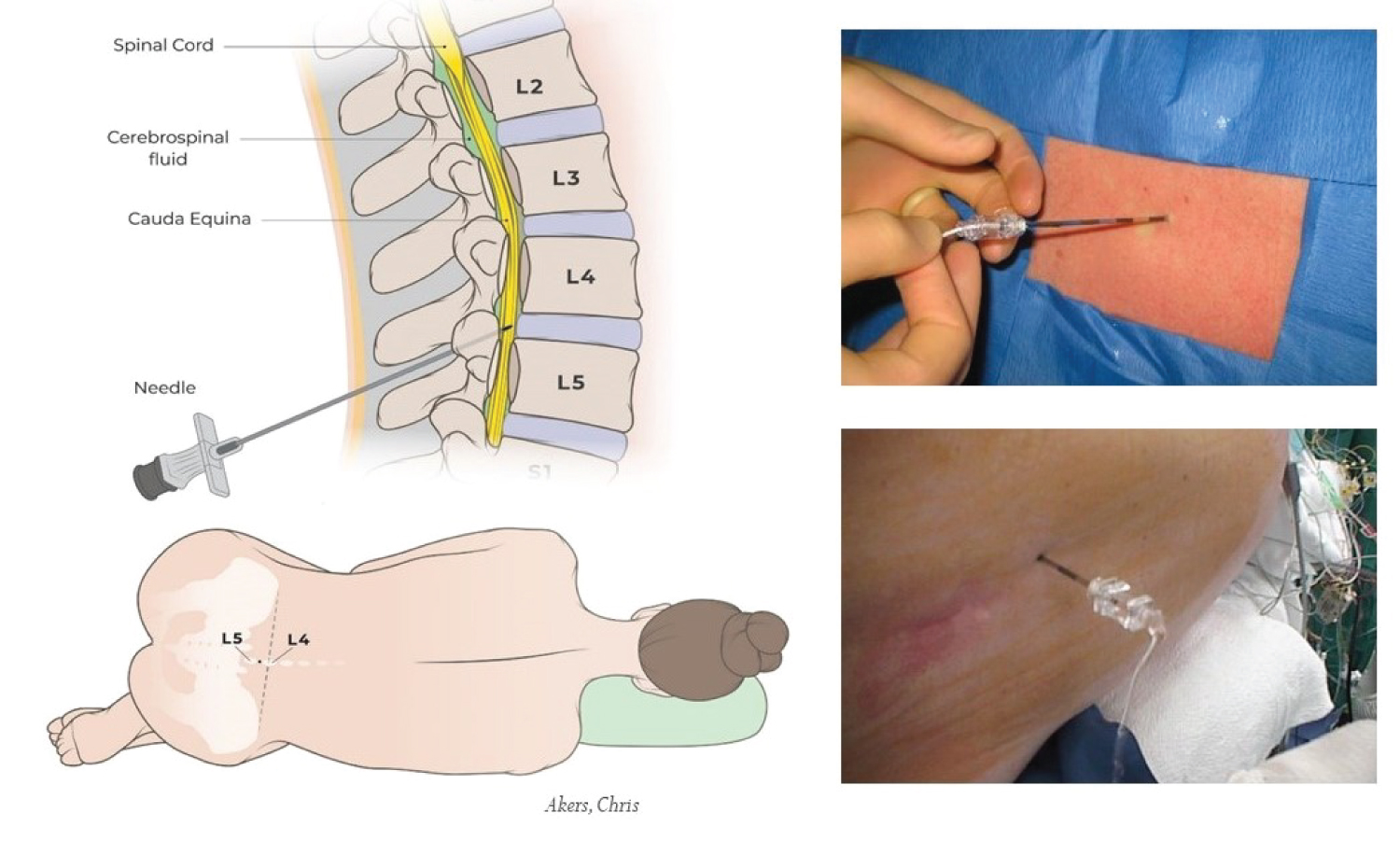
Figure 2:
Position and entry point for lumber CSF drainage.
A CSFd catheter is placed under general anesthesia. The lateral decubitus with fetal position is better in lumbar flexion to open the intervertebral space. The lateral decubitus position has lower hydrostatic pressure of the column of CSF compared to the sitting position. The midline approach is preferred instead of the paramedian line approach to avoid injury of the epidural venous plexus and bleeding and soft tissue injuries. The L3-4 and L4-5 intervertebral spaces are preferred to avoid injury of the extension lumbar spinal cord.
For post-lumbar, scoliosis and elderly patients with lumbar spinal fusion, lumbar image studies may help to assess the level intervertebral space and determine the angle and direction chosen during the CSFd catheter insertion. Ultrasound is one of the noninvasive imaging methods that may help with CSFd placement. CSFd catheter placement under general anesthesia, L3-4, L4-5, or even L5-S1 intervertebral space, is preferred to avoid injury of the extension lumbar spinal cord. For the same reasons, L2-3 may be safe with senior patients or patients with clear image studies that prove the end of the lumbar spinal cord is above the L2 level (Figure 2).
The 80-cm, barium-impregnated silicone drainage catheter with a closed radiopaque tip is inserted 6-8 cm into the subarachnoid space after 14G Tuohy stainless steel needle is successfully inserted. The drain catheter should be secured with a taped dressing to prevent it from being pulled out, disconnected, or migrated with patient movement. After CSFd systems are connected to the pressure transducer measurements, documentations of open lumber-CSFP (ICP) pressure and associated hemodynamic paramagnets, such as blood pressure, CVP, and pulmonary artery pressure, are important as reference data for further management of CSFd. Dynamic observation changes of ICP/CSFP with hemodynamic parameters are more important. It is critical to remember to close the high-pressure primer normal saline in the CSF transducer system before opening to the patient in order to measure ICP. If the primer normal saline is under high pressure (250-300 mmHg) from an opening, there is a high risk of primer solution retrograde pushed into subarachnoid space, resulting in increased ICP and worsening spinal cord perfusion.
Setting up and positioning of CSFd
How to position of CSFd systems (Figure 3) is another important concept in the set-up process (Figure 4). Controlled CSFd pressure should be set up with a goal of ICP level of 10 mmHg about zero level on transducer for CSFP monitoring, or at least below the CVP level if the patient’s open ICP is higher than CVP. The benefit of this setting can be controllable on this system, both in volume collection and speed of CSF drainage, to reach our goal of ICP < 10 mmHg without increased risk of causing bloody CSF-since bloody CSF is most likely due to sudden change subarachnoid space pressure. We avoid placing CSFd chamber under the operation bed close to the floor or below zero.
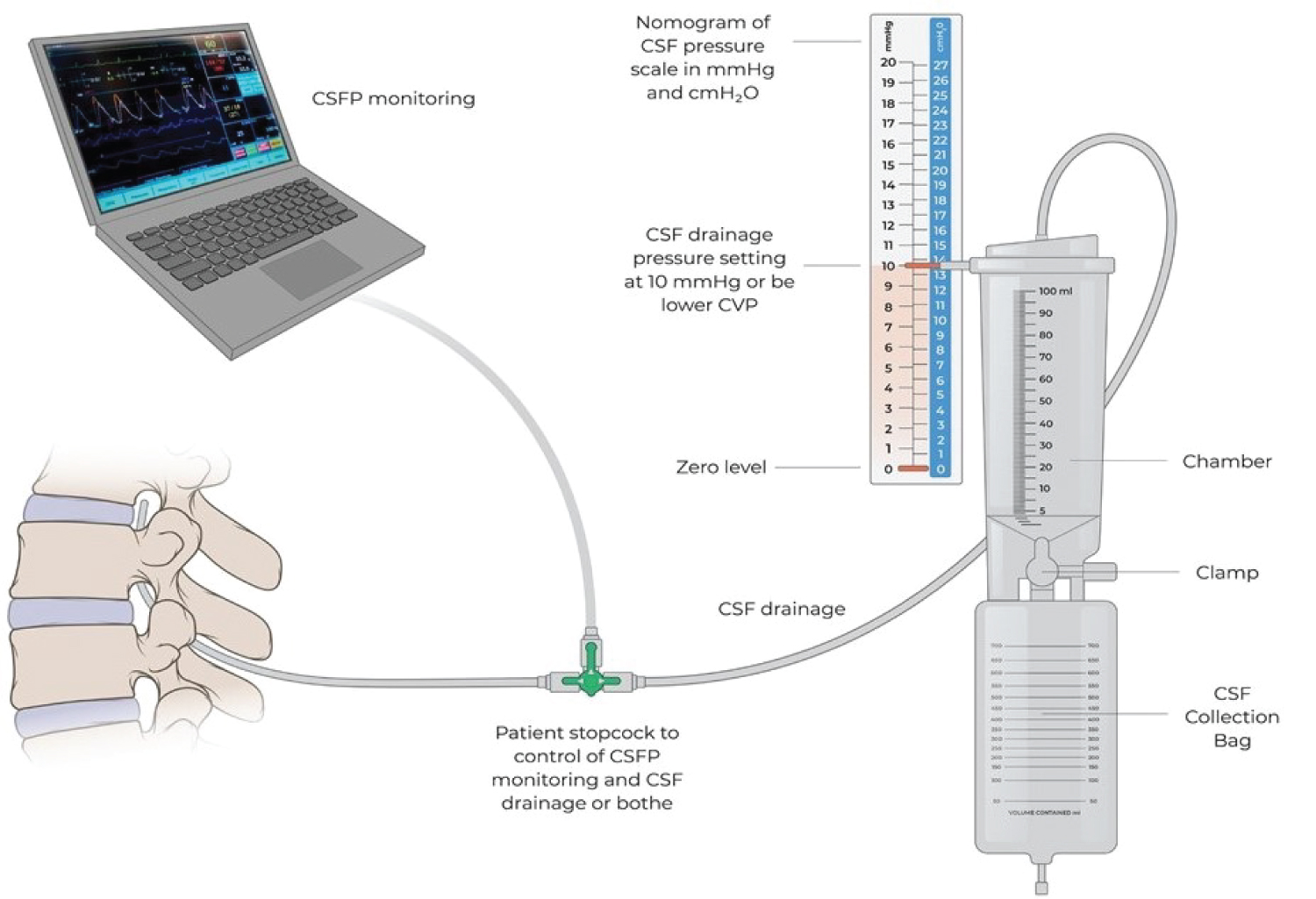
Figure 3:
External CSF drainage system.
Transducer zeroing position for ICP monitoring is at the level of the right atrium. CSF drainage pressure is set up with the goal of reaching an ICP level of 10 mmHg.

Figure 4:
Setting up and position of CSF draining systems.
Primer transducer tube system with 0.9% normal saline instead of heparin-contained solution. The transducer position at the level of the right atrium with supine and the lateral decubitus position intraoperatively. At this position, the head and lumber spinal cord is at the same level (ICP = LCSFP). The transducer position should be at the level of the CSFd catheter insertion point for acutely measured lumber CSFP at 15-30 degree sitting position in ICU (ICP < LCSFP).
Intraoperative management of CSFd
There is no standardized protocol -including timing, duration, and volume- for CSFd use and the decision varies between anesthesiology teams (Figure 5). It also varies among patients’ medical condition and location of disease, as well as the complexity of the surgery. In our institution, after recording baseline ICP and hemodynamic parameters, continuous monitoring ICP with intermittent drainage of CSF begins. Intraoperative responses also depend on the patient’s baseline conditions, open ICP, risk of spinal cord ischemic from surgery, and intraoperative change in CVP. To prevent complications related to over-drainage of CSF, controlled CSFd is also important. Too fast of a change in ICP is very dangerous as it not only will induce bloody CSF but also can cause ICH and even herniation, with lethal complications.
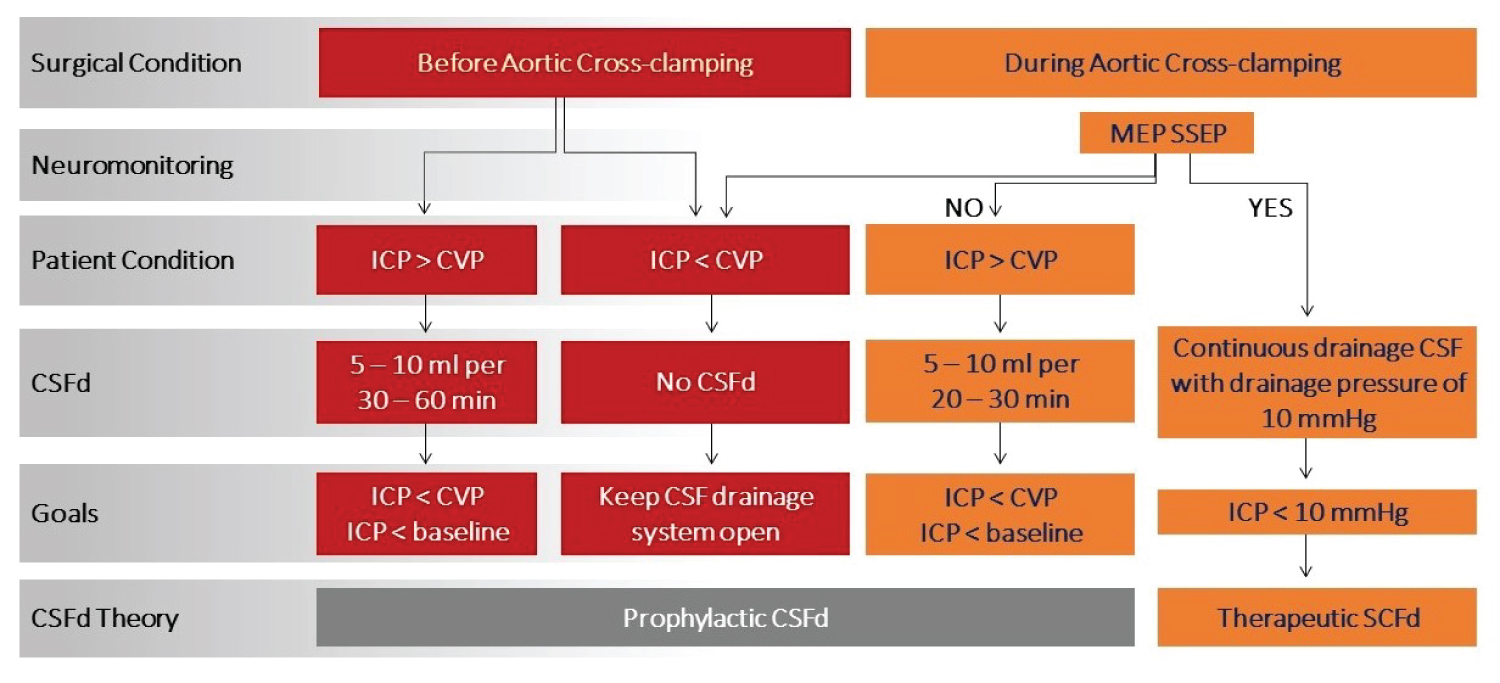
Figure 5:
Intraoperative neuromonitoring guided CSF drainage flow chart.
CSFd: Cerebrospinal Fluid drainage; CVP: Central Venous Pressure; MEP: Motor Evoked Potentials; SSEP: Somatosensory Evoked Potentials.
Before heparin is given
Continuous monitoring with intermittent drainage to keep the CSFd system open if ICP is below CVP or 10 mmHg by using the patient stopcock to close the draining line and open the patient line in order to transmit the ICP from the lumbar CSF catheter to the transducer. If ICP is higher than CVP or 10 mmHg, we prefer to remove 5-10 ml of CSF every 30-60 minutes before heparin is given. The goal is to reduce ICP to less CVP by removing CSF and all other variable that increase CVP.
After aortic cross-clamping
An increase in CSF pressure after aortic cross-clamping is due to volume changes in the capacitance veins of the epidural space. Therefore, CSF drainage will lower the intrathecal volume and pressure to improve spinal cord perfusion. Normal CSFd may be needed during this period as 5-10 ml every 20-30 minutes. The goal is to ensure ICP is lower than CVP. On the other hand, maintaining distal aortic blood flow and pressure by controlling distal pump perfusion increases spinal cord perfusion, and reduces spinal cord ischemic injury.
Loose neuromonitoring signals
Appropriate neuromonitoring provides sensitive information on spinal cord perfusion status. Intraoperative change or loss of neuromonitoring signals by MEP suggests spinal cord ischemia during DTAA and TAAA repair. Estrera and colleagues [6] use SSEP and MEP during TAAA repair as a guide in reattaching intercostal arteries for increasing spinal cord perfusion by means of the collateral network of the spinal cord. We are using neuromonitoring-guided CSF drainage intraoperatively (Figure 5). If a change or loss of MEP and SSEP occurs, CSF must be drained more aggressively to reduce ICP and improve spinal cord perfusion, combined with maintaining hemodynamic stability. Traditionally, a controlled, continuous CSFd begins while ICP monitoring is continued (Figure 3). CSFd pressure is controlled by setting up a goal ICP pressure above zero point and below CVP until MEP and SSEP signals return.
Other important factors
Maintaining hemodynamic stability of adequate cardiac output, blood pressure for organ perfusion and stable circulation volume status, efficient red blood volume for oxygen carry capacity, and avoiding acidosis, alkalosis, and electrolytic imbalance are all very important contributions to adequate spinal cord perfusion.
Postoperative management of CSFd
Postoperative management includes multidisciplinary strategies to prevent spinal cord ischemic injury. CSFd is usually coupled with hemodynamic parameters. Under ideal conditions -including hemodynamic stability, acquit of oxygen carrier and delivery- postoperative management of CSFd is mainly dependent on the patient’s neurological examination and intraoperative changes in neuromonitoring (Figure 6). Changes in SSEP and/or MEP imply that immediate or delayed neurologic deficits may occur in the perioperative period. When there are no intraoperative changes in SSEP and MEP and no postoperative neurologic deficits occur, the only thing required is keeping the CSFd system open. The risk of SCI in those patients is much lower and CSFd is more preventive than for treatment of spinal cord ischemia.
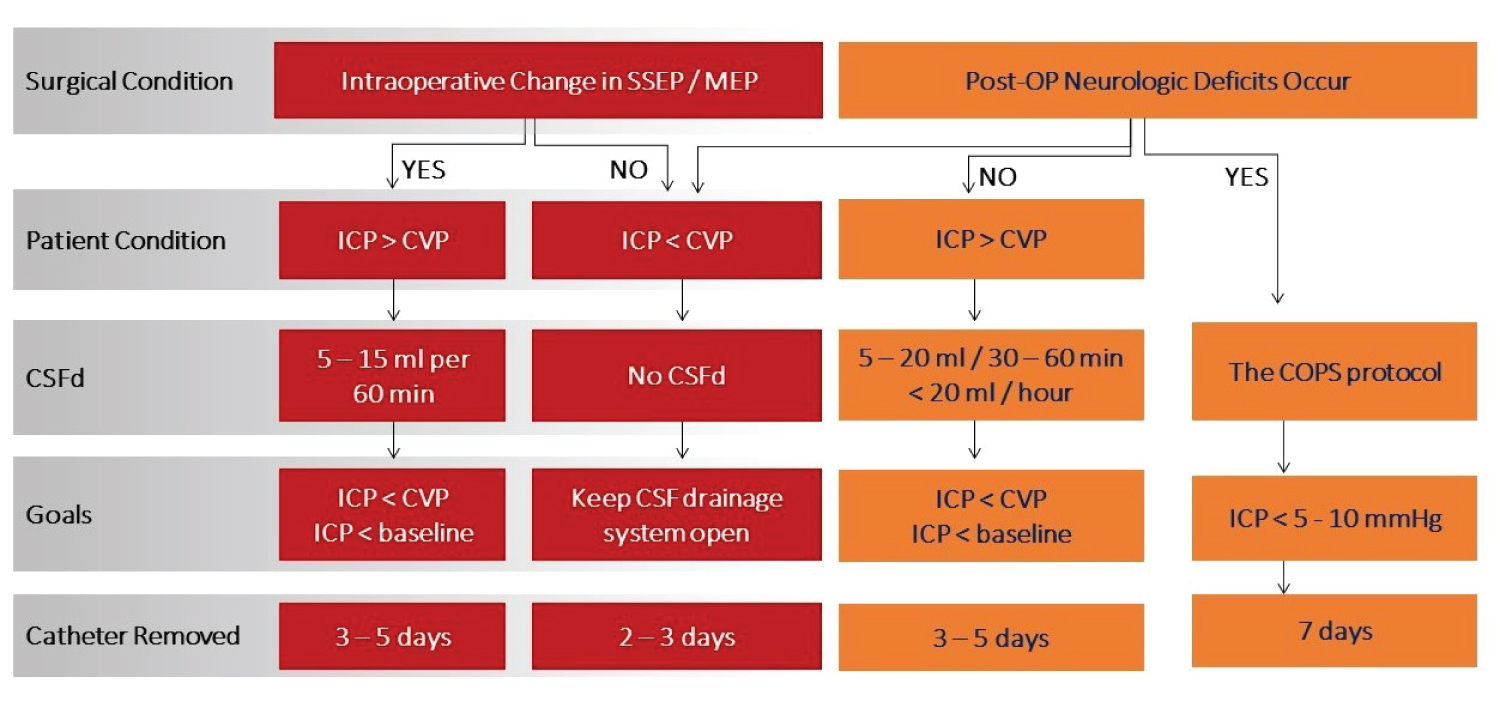
Figure 6:
Postoperative management of CSF drainage flow chart.
CSFd: Cerebrospinal Fluid drainage; CVP: Central Venous Pressure; ICP: Intracranial Pressure; MEP: Motor Evoked Potentials; SSEP: Somatosensory Evoked Potentials.
However, when neurological deficits occur postoperatively, CSFd is an important therapeutic approach in collaboration with increasing spinal cord blood supply and oxygen delivery. Figure 6 introduces postoperative CSFd management in detail to respond to changes in neuromonitoring intraoperatively and postoperative neurologic deficits. If reported previously, if neurologic deficits occur, CSFd is recommended to achieve a pressure of < 5-10 mmHg, in addition to blood transfusing to keep hemoglobin > 10-12 g/dl, cardiac index > 2.5 l/min BSA and blood pressure of 90-100 mmHg (COPS protocol). Delayed paraplegia can occur days after the surgery as a result of spinal cord ischemia, which could be caused by postoperative hypotension, spinal cord edema, reperfusion injury, and intercostal artery thrombosis. At this time, we start the COPS protocol (Figure 7).
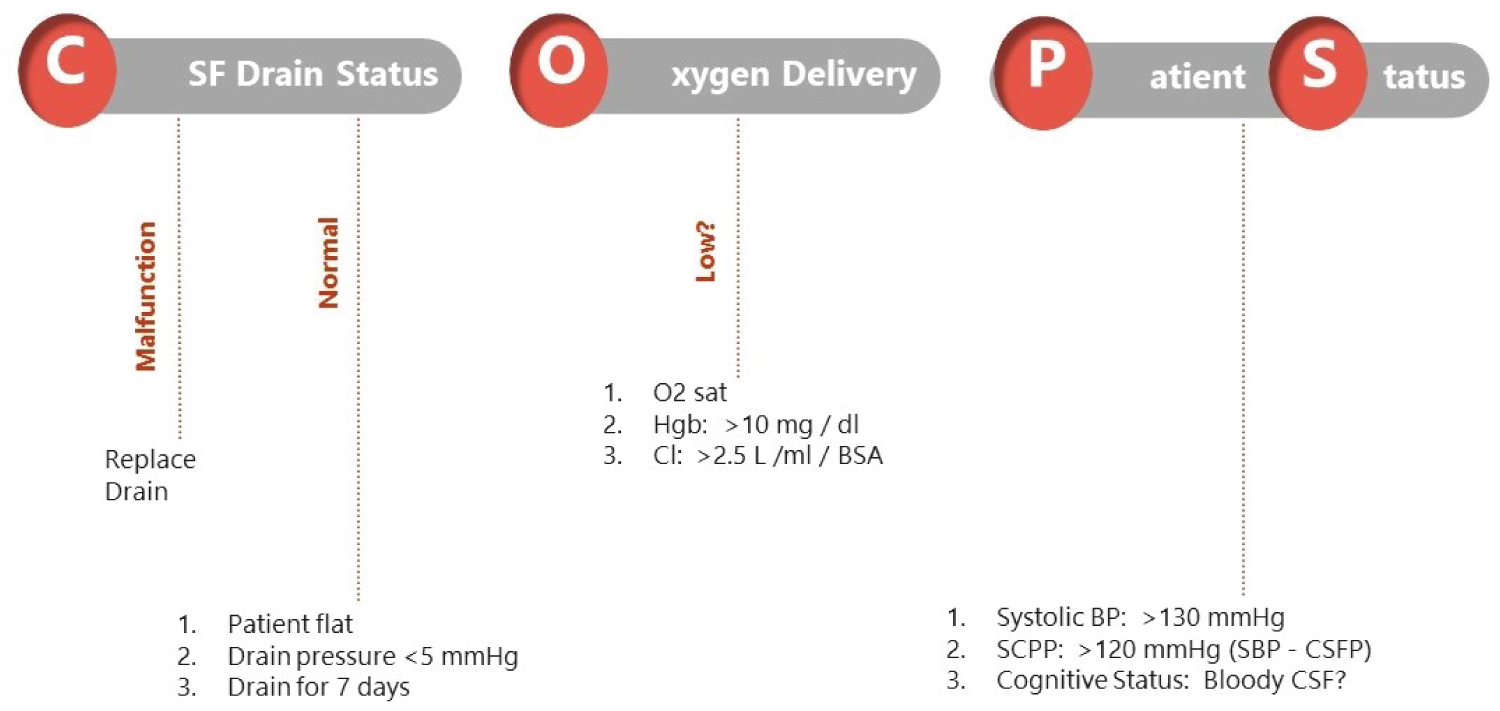
Figure 7:
Postoperative management of CSF drainage: Treating delayed spinal cord ischemia.
Treatment for delayed neurologic deficit: The COPS protocol for managing CSFD.
BP: Blood Pressure; BSA: Body Surface Area; CI: Cardiac Index; CSF: Cerebrospinal Fluid; CSFP: Cerebrospinal Fluid Pressure; Hgb: Hemoglobin; MAP: Mean Arterial Pressure; SCPP: Spinal Cord Perfusion Pressure.
Aggressive drainage of CSF to reduce ICP, and increased SCPP is routine. Unfortunately, the lack of limitations in volume and rate of drainage of CSF to lower ICP may increase the risk of bloody CSF and associated neurologic complications.
Management of most common complication of CSFd
The most common perioperative complications related to CSFd in patients undergoing DTAA and TAAA repair are bloody CSF or malfunctioned CSFd system, non-clinically significant subdural hematoma postoperatively and post-puncture headache. However, subdural hemorrhagic or bloody CSF with neurologic changes and meningitis are rare but may be lethal [8,9]. In this situation, magnetic resonance imaging or computed tomography scan is needed and neurosurgeons are often asked to consult for continued management.
CSF drainage associated ICH or bloody CSF
Strategies that control the volume and speed of spinal fluid drainage while at the same time achieving the goal of increasing SCPP are helpful in preventing and reducing serious complications related to CSF over drainage. Patients with cerebral atrophy are at increased risk for complications of spinal fluid drainage [17]. From our clinical observation, bloody CSFd often occurs when aggressive and excessive CSFd is required to achieve CSF pressures < 5-10 mmHg during the therapeutic CSFd period. The CSF volume removed is not directly associated bloody CSF during the earlier period. In some cases, CSF can be removed over 200 ml over 24 hours without problem. However, 10-20 ml of CSFd has shown bloody CSF in some patients. The benefits of spinal cord protection vs. the increased risk of subdural hemorrhage are sometimes difficult to balance. Multidisciplinary discussion and individual case management are required.
Once bloody CSF is noted in the draining system, CSFd should cease immediately by clamp patient stopcock (Figure 3). Careful neurologic examination should be performed and coagulation studies should be sent. Correction of coagulation abnormalities must be done as soon as possible. For clinically asymptomatic patients, negative CT/MRI studies for bloody CSF and with relative normal coagulation test, CSFd can be restarted after 4-6 hours, as needed, for spinal cord protection. The team must be careful to do a test open CSFd before starting to follow the CSFd protocol (Figure 8). This can be tricky when the patient’s CSFd appears bloody with ICH and, at the same time, the patient develops weakness in the lower extremities. When this occurs, rescuing the patient is top priority. If ICH is stable after a period time, test opening CSFd can be attempted and CSFd followed if there is no bloody CSF in the drainage system (Figure 9). In our practice, we believe best results come from balancing risks and benefits for patients by using our clinical knowledge and experience along with multidisciplinary discussions.
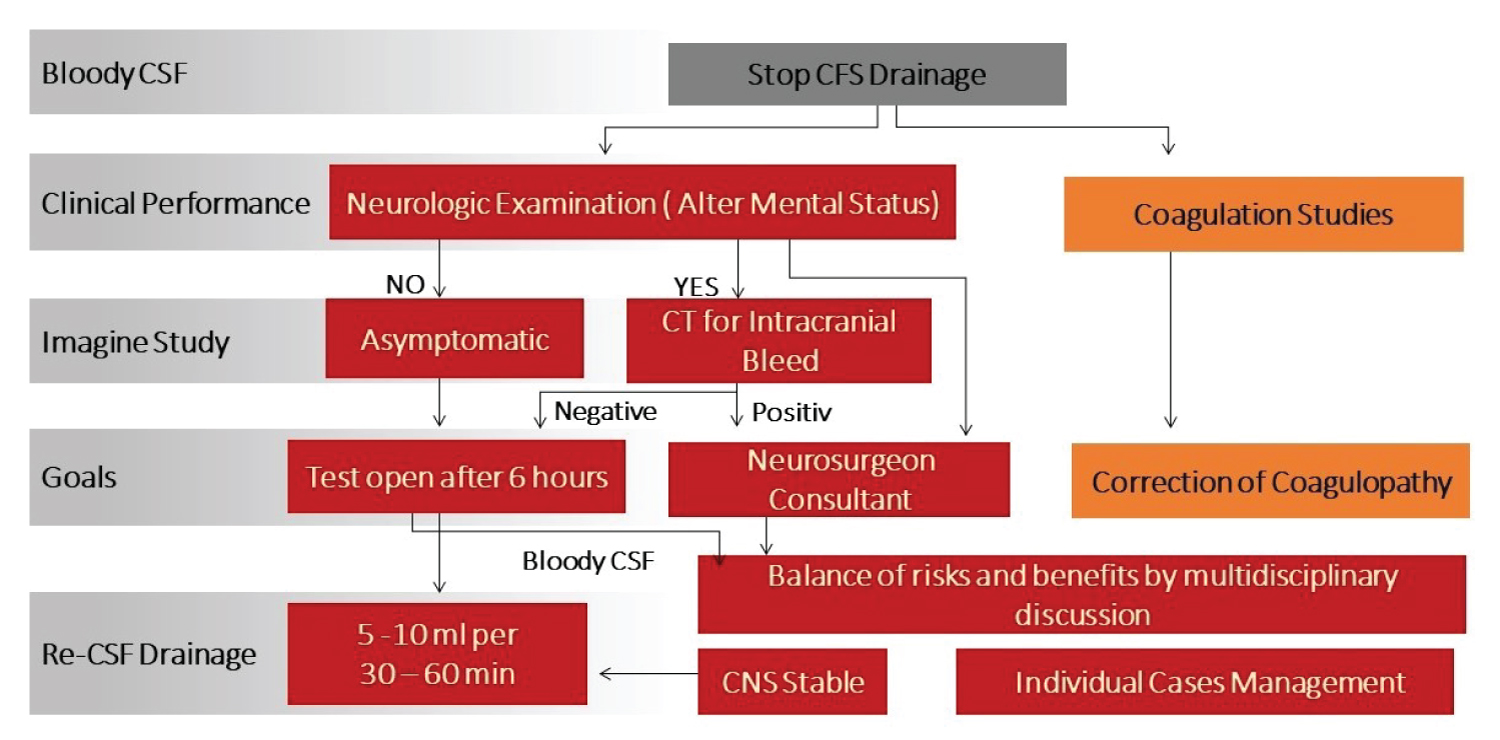
Figure 8:
Management of bloody CSF drainage flow chart.
CSF: Cerebrospinal Fluid; CNS: Central Nervous System
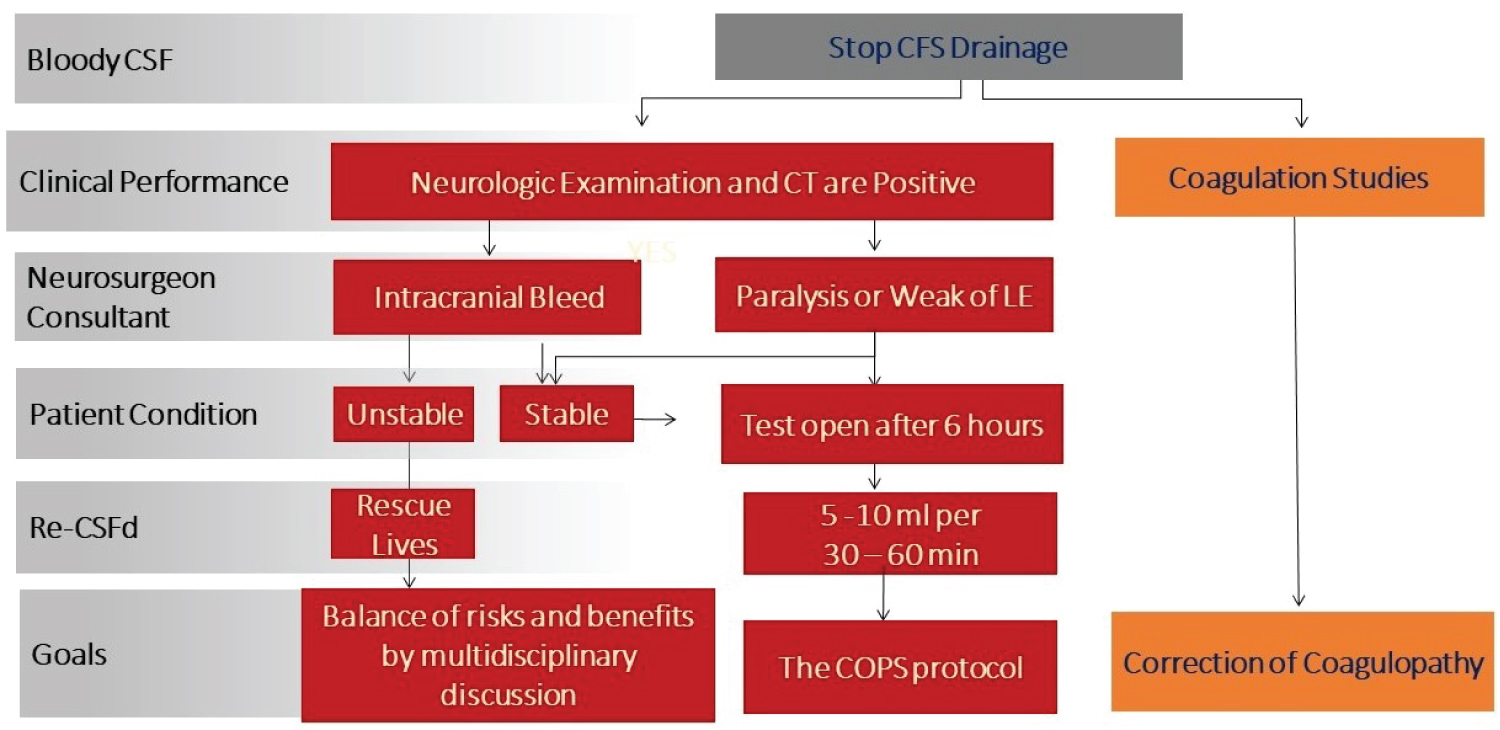
Figure 9:
Management of bloody CSF drainage with spinal cord ischemia flow chart.
CSF: Cerebrospinal Fluid; LE: Lower Extremities.
Management of post-dural puncture headache, with and without CSF leak
Headache due to intracranial hypotension by a reduction in CSF volume or intracranial hypotension may lead to cerebral and meningeal vasodilation, which may cause headache, characterized by post-dural, with some nausea. It can also accompany other neurological complications, including ocular and auditory problems. It is the most common complication associated with CSFd with and without CSF leak. Conservative treatment is preferred, including lying flat bed rest from 8-24 hours and oral or IV hydration. Lumbar insertion should be applied with local pressure dressing if CSF leak is present. Oral analgesic medication, such as nonsteroidal, anti-inflammatory drugs and weak opioids may help release symptoms. Caffeine is beneficial in this setting for cerebral vasoconstriction and increased CSF production. Patients can receive intravenous caffeine of 500 mg if it cannot be taken orally [18]. If headache persists after 24-48 hours of conservative therapy -or with persistent CSF leak over 24 hours- an autologous-blood patch will be needed.
Malfunction or post-bloody CSFd occlusion of the drainage catheter
This condition occurs quite often during the postoperative period in ICU. The CSFd system should be troubleshot carefully. The problems of external catheter blockage, fracture, folding jam, and filter occlusion may be resolved without the need for a new paced CSFd catheter. Otherwise, reinsertion of CSFd catheter is necessary when CSFd is needed for spinal cord protection. Coagulation status needs to be evaluated before the new insertion.
Conclusion
There are no standard protocols for perioperative management of CSFd in TAAA and DAA patients. In addition, the handling of CSFd is dependent on the rapid change of the patient’s condition and clinical performance, risk from the diseases itself, and complexity of surgery and aortic team consensus. This article introduces more than 25 years of clinical experience from our aortic team. Further studies using neuromonitoring-guided intraoperative CSFd in TAAA and DTAA repairs are needed.
Disclosures
Dr. Estrera is a consultant for WL Gore, CryoLife, Edwards Lifesciences, and Terumo Aortic. The other authors have no conflicts of interest and no funding was obtained for this study.
References
- Melo RGE, Duarte GS, Lopes A, Alves M, Caldeira D, et al. Incidence and prevalence of thoracic aortic aneurysms: A systematic review and meta-analysis of population-based studies. Semin Thorac Cardiovasc Surg. 2022 Spring;34(1):1-16.
- Olsson C, Thelin S, Stahle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: Increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114:2611-8.
- Coselli JS, LeMaire SA, Miller CC 3rd, Schmittling ZC, Koksoy CJ, et al. Mortality and paraplegia after thoracoabdominal aortic aneurysm repair: a risk factor analysis. Ann Thorac Surg 2000;69:409-14.
- McGarvey ML, Cheung AT, Szeto W, Messe SR. Management of neurologic complications of thoracic aortic surgery. J Clin Neurophysiol. 2007;24:336-43.
- Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, et al. 2010 ACCF/AHA/AATS/ ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266-369.
- Estrera AL, Sheinbaum R, Miller CC 3rd, Harrison R, Safi HJ. Neuromonitor-guided repair of thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg. 2010;140 (6 Suppl):S131-5; discussion S142-6.
- Rong LQ, Kamel MK, Rahouma M, White RS, Lichtman AD, et al. Cerebrospinal-fluid drain-related complications in patients undergoing open and endovascular repairs of thoracic and thoraco-abdominal aortic pathologies: A systematic review and meta-analysis. Br J Anaesth. 2018;120:904-13.
- Estrera AL, Sheinbaum R, Miller CC 3rd, Azizzadeh A, Walker JC, et al. Cerebrospinal fluid drainage during thoracic aortic repair: safety and current management. Ann Thorac Surg. 2009;88:9-15.
- Abdelbaky M, Papanikolaou D, Zafar MA, Ellauzi H, Shaikh M, et al. Safety of perioperative cerebrospinal fluid drain as a protective strategy during descending and thoracoabdominal open aortic repair. JTCVS Techniques. 2021; Volume 6, Number C p. 1-8.
- Lindeire S, Hauser JM. Anatomy, back, artery of Adamkiewicz. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan. 2023 Jul 25.
- Griepp RB, Griepp EB. Spinal cord perfusion and protection during descending thoracic and thoracoabdominal aortic surgery: The collateral network concept. Ann Thorac Surg. 2007 Feb;83(2):S865-9; discussion S890-2.
- Etz CD, Kari FA, Mueller CS, Brenner RM, Lin HM, et al. The collateral network concept: remodeling of the arterial collateral network after experimental segmental artery. J Thorac Cardiovasc Surg. 2011 Apr;141(4):1029-36.
- Arora L, Hosn MA. Spinal cord perfusion protection for thoraco-abdominal aortic aneurysm surgery. Curr Opin Anaesthesiol. 2019 Feb;32(1):72-79.
- Rashid S, Hamid M, Malick SA. The use of lumbar drain for spinal cord protection in a thoraco abdominal aortic aneurysm surgery. J Coll Physicians Surg Pak. 2022 Aug;32(8):S195-S196.
- Cambria RP, Clouse WD, Davison JK, Dunn PF, Corey M, et al. Thoracoabdominal aneurysm repair: results with 337 operations performed over a 15-year interval. Ann Surg 2002;236:471-9; discussion 479.
- Oftadeh M, Ural N, LeVan P, Prabhu V, Haske M. The evolution and future of spinal drains for thoracic aortic aneurysm repair: A review. J Cardiothorac Vasc Anesth. 2021 Nov;35(11):3362-3373.
- Wynn MM, Mell MW, Tefera G, Hoch JR, Acher CW. Complications of spinal fluid drainage in thoracoabdominal aortic aneurysm repair: A report of 486 patients treated from 1987 to 2008. J Vasc Surg. 2009 Jan;49(1):29-34; discussion 34-5.
- Russell R, Laxton C, Lucas DN, Niewiarowski J, Scrutton M, et al. Treatment of obstetric post-dural puncture headache. Part 1: conservative and pharmacological management. Int J Obstet Anesth. 2019 May;38:93-103.