Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/134
Review Article Open Access
How Does the SARS-CoV-2 Pandemic Affect Cardiac Surgery Practice
Benjamin Morey, MD, Hong Liu, MD* and David Li, MD*
Department of Anesthesiology and Pain Medicine, University of California Davis Health System, Sacramento, CA, USA
David Li, MD and Hong Liu, MD, FSAE, Department of Anesthesiology and Pain Medicine, University of California Davis Health, 4150 V Street, Suite 1200, Sacramento, CA 95817, USA, Tel: 916-734-5031, Fax: 916-734-7980, E-mails: dpli@ucdavis.edu; hualiu@ucdavis.edu
Editor: Henry Liu, MD, MS, FASA, Professor of Anesthesiology, Department of Anesthesiology and Perioperative Medicine, Penn State Milton S.Hershey Medical Center, 500 University Drive, PO Box 850, Mail code H187, Hershey, PA 17033-0850, USA; Tel: 717-531-5167; Fax 717-531-7790; E-mail: henryliupa@gmail.com
Received: December 06, 2020 | Accepted: January 01, 2020 | Published: January 04, 2020
Citation: Morey B, Liu H, Li D. How Does the SARS-CoV-2 Pandemic Affect Cardiac Surgery Practice. Transl Perioper & Pain Med 2021; 8(1):315-321.
Abstract
The World Health Organization declared the coronavirus disease 2019 (COVID-19) or severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as a pandemic in March 2020, and it had a significant impact on health care systems globally. Each country responded to COVID-19 differently, however, this was done broadly by fortifying and prioritizing health care provision as well as introducing social lockdown in order to contain the infection and minimizing the risk of transmission. Since SARS-CoV-2 affects multiple organs, such as heart, lungs and kidneys, etc. and adversely affects the surgical outcomes, elective surgeries, especially cardiac surgery have been rescheduled because the cardiac surgical patient population was a particularly high-risk group that would require a massive level of valuable resource allocation and concrete workflow adjustment. Right now, healthcare facilities worldwide are well-served continuing to use multi-disciplinary approaches to address the cardiac surgical workload in a manner that is safe for both patients and providers, and effectual in the preservation of scarce health care resources.
Keywords
COVID-19, SARS-CoV-2, Cardiac surgery
Introduction
The ongoing pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to a global health crisis on a scale not seen since the 1918 influenza pandemic. At the time of writing, 55,928,327 cases and 1,344,003 deaths have been reported to the World Health Organization [1]. The scale of the problem has pushed healthcare systems worldwide to the brink as we attempt to diagnose and treat new cases, prevent the spread of the virus within the community, protect frontline healthcare workers, and reasonably manage those patients who require care unrelated to the pandemic. Our institution saw the first case of community-acquired SARS-CoV-2 in the United States [2], and shortly thereafter elective surgical procedures were curtailed significantly. Cardiac surgical services were especially scrutinized given its attendant consumption of resources that were scarce during this influx of critically ill patients. Here, we will discuss the clinical considerations of the SARS-CoV-2 virus on the cardiac surgical population, the approach towards delaying versus proceeding with cases, the allocation of vital hospital resources, and how to best manage the growing backlog of patients who will require surgical intervention in the future.
The Effects of SARS-CoV-2 on the Cardiovascular and Pulmonary Systems
Patients with pre-existing cardiovascular disease have been identified as a particularly high-risk group with respect to SARS-CoV-2 due to the virus' multi-organ impact (Table 1). Early in the course of the pandemic, those with pre-existing cardiovascular conditions were demonstrated to have higher mortality rates [3,4]. However, there is now a growing body of literature documenting the associated cardiovascular effects of the virus including myocardial injury, heart failure, arrhythmias, and vascular thrombosis [5]. Myocardial injury evidenced by elevated troponin levels in hospitalized patients has been associated with higher early mortality [6,7]. Those patients who have been identified as higher risk of developing myocardial injury tend to be elderly and have preexisting hypertension, heart failure, coronary artery disease (CAD), and diabetes [3]. Further, patients who developed myocardial injury were more likely to suffer malignant arrhythmias and had elevated levels of multiple inflammatory biomarkers suggesting a potential role for severe, systemic inflammation as a contributor to the myocardial injury observed [7]. Particular attention has been paid to the role of angiotensin-converting enzyme 2 (ACE2) which is a receptor for SARS-CoV-2. Given that ACE2 is found in high quantities within the myocardium, signaling pathways related to this enzyme may play a role in SARS-CoV-2-related myocardial injury [8].
Table 1: SARS-CoV-2 and its multi-organ impact.
|
Organ system |
Areas of Impact |
Clinical Consequences |
|
Cardiovascular |
-Myocardial injury -Heart failure -Arrhythmias -Vascular thrombosis |
-Elevated cardiac biomarkers -Hemodynamic instability -Need for prolonged periods of inotropic support and potentially mechanical assist devices -Increased perioperative bleeding, heparin resistance, heparin-induced thrombocytopenia |
|
Pulmonary |
-Hypoxemic respiratory failure -Ventilation-perfusion mismatch -Respiratory acidosis |
-Prolonged post-op ventilation -Possible tracheostomy if unable to be weaned from ventilator -Need for ECMO |
|
Renal |
-Renal failure |
-Need for post-op renal replacement therapy |
|
Others |
-Immune dysregulation |
-Elevated inflammatory biomarkers -Persistent fever |
Note: HIT: Heparin induced thrombocytopenia; V/Q: Ventilation-perfusion ratio; ECMO: Extracorporeal membrane oxygenation; Post-op: Post-operative
The prothrombotic state associated with the virus has also been well-documented. One study described 16% of hospitalized patients experiencing thrombotic events, which was independently associated with increased mortality [9]. Risk factors for thrombosis included CAD, prior myocardial infarction, and higher D-dimer levels on presentation [9]. Subsequently, unless there is a contraindication, hospitalized patients with SARS-CoV-2 should be receiving prophylactic anticoagulation [10]. A small trial has demonstrated improved gas exchange and decreased need for mechanical ventilation when SARS-CoV-2 patients were administered therapeutic levels of anticoagulation, and larger trials are currently underway to better evaluate this [11]. This has clear implications for the cardiac surgical population for whom physicians may encounter an increased risk of intraoperative bleeding, heparin resistance, heparin-induced thrombocytopenia, and thrombosis of mechanical circulatory support devices [5].
The pulmonary complications associated with SARS-CoV-2 also carry significant weight in the perioperative management of cardiac surgical patients. During the early stages of the pandemic patients presented with symptoms consistent with a viral pneumonia and had imaging demonstrating bilateral lung consolidations or ground-glass-opacities [12]. The most severe manifestation of the infection is a severe hypoxemic respiratory failure frequently necessitating supplementary oxygen and mechanical ventilation. These patients are typically managed with a "lung-protective" ventilatory technique utilizing lower tidal volumes, positive end-expiratory pressure (PEEP), and minimization of airway pressures [13]. The impact of using these ventilatory parameters is as of yet unquantifiable, but could have a profound impact on the outcomes of the cardiac surgical population. For example, patients with significant cardiovascular dysfunction in the perioperative period may not be able to tolerate the respiratory acidosis that can develop with permissive hypercarbia. Likewise, elevated intrathoracic pressures from the use of PEEP may be detrimental for patients with lower cardiac output. Finally, augmenting ventilation-perfusion matching via prone positioning may be problematic in a patient population with multiple lines, tubes, and healing chest wall incisions postoperatively.
Outcomes of Patients Undergoing Cardiac Surgery with SARS-CoV-2
There is a paucity of evidence available describing the outcomes of patients who undergo cardiac surgery with SARS-CoV-2. However, there are a few retrospective studies evaluating outcomes in surgical patients who were known to have SARS-CoV-2. Lei and colleagues examined 34 such patients [14]. 44% of these patients required intensive care unit (ICU) admission compared to 25% of hospitalized patients who did not undergo surgery [14]. The mortality rate amongst the surgical cohort was 21% compared to the 2-3% in non-surgical patients at that time [14]. Another group compared propensity-matched patients undergoing cardiac surgery pre-pandemic to those undergoing cardiac surgery with asymptomatic SARS-CoV-2 infections and found that the patients with SARS-CoV-2 had higher rates of ICU readmission and mortality [15]. In an analysis of 1128 patients undergoing surgery with confirmed SARS-CoV-2 infection, approximately 50% had postoperative pulmonary complications which were strongly associated with higher mortality [16]. The groups that were highlighted as being at particular risk included several that are frequently amongst the cardiac surgical cohort: Males, age > 70, American Society of Anesthesiologists (ASA) class 3-5, emergency surgery, and major surgery [16]. For those reasons, it is not unexpected that patients undergoing cardiac surgery would be especially-susceptible to the effects of SARS-CoV-2. Cardiopulmonary bypass itself is known to induce a significant inflammatory response. This could exacerbate or render patients more susceptible to the deleterious effects of SARS-CoV-2, for which a significant component of the pathophysiology is thought to itself stem from a robust inflammatory response [17]. Further, as SARS-CoV-2 produces primarily respiratory effects, surgical interventions that significantly alter pulmonary mechanics and are themselves associated with multiple respiratory complications are best-avoided whenever possible [18,19].
Changes to Operating Room throughout during the Pandemic
Our institution reduced elective cardiac surgical case volume significantly in March of 2020 with the initial spike in SARS-CoV-2 cases (Figure 1). This was in accordance with actions undertaken by most facilities in the United States and worldwide. In a survey of 67 centers that performed adult cardiac surgery in North America, case volume declined to 45% of baseline [20]. However, in both North America and worldwide there was no significant difference in terms of case volume reduction between areas of high and low SARS-CoV-2 burden [20,21]. Multiple challenges with regards to cardiac surgical case selection followed this decision, the first and foremost being how to discern which cases were medically essential enough to expose high-risk patients to an environment where they could potentially become infected with SARS-CoV-2. Likewise, there was also difficulty establishing which cases could be safely deferred to a later date without risking increased cardiovascular morbidity.
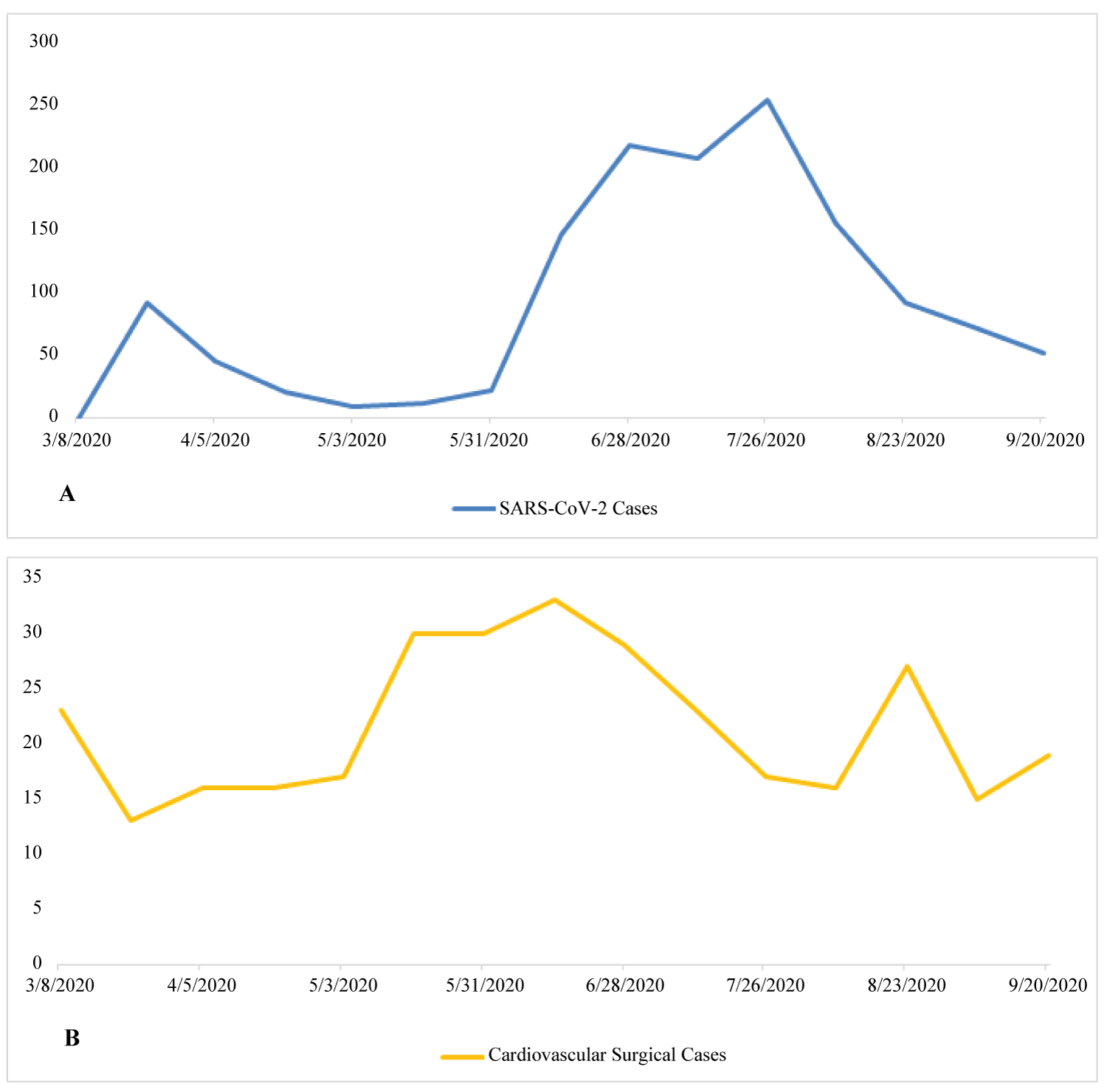
Figure 1: (A) Trend of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cases at one institution over time during coronavirus disease 2019 (COVID-19) pandemic. The Y-axis is the number of COVID-19 patents; (B) The cardiovascular cases performed during the same period of time. The Y-axis is the number of cardiovascular cases.
Most institutions, including our own, adopted a tiered approach depending on the individual institution's inpatient SARS-CoV-2 burden as was recommended by the Society of Thoracic Surgeons [22]. Similar guidelines from Canada and the United Kingdom exist [23,24]. These approaches focus on deferring all but emergent procedures such as repair of type A aortic dissections during periods of high SARS-CoV-2 burden, and then gradually including other patients as cases subside. Patients with high-risk pathology (e.g. severe, symptomatic aortic stenosis, left main CAD, large or rapidly-expanding aortic aneurysms) were the first to be recommended for operations when the SARS-CoV-2 burden lessened. Throughout, any patients thought to be high-risk for poor outcomes should they contract SARS-CoV-2 (elderly, frail, other significant co-morbidities, etc.) were deferred if deemed safe from a cardiovascular standpoint. Likewise, those requiring operations that would require prolonged post-operative ICU management or blood product administration (both involving significant use of scarce resources) were also deferred whenever it was felt to be safe.
Once the SARS-CoV-2 burden had subsided in our institution, we began scheduling higher numbers of elective cardiac cases (Figure 1). Following this, several additional challenges arose. Whereas pre-pandemic there could be a quick turn-around time from diagnosis to surgical intervention, now patients were scheduled on the basis of waiting-time and disease-acuity. Guidelines such as those provided by the European Society of Cardiology (ESC) were referenced prior to the scheduling of cardiac surgical cases [25]. In the guidelines provided by the ESC, cases were divided into 4 groups: Emergent, urgent - performed within days, lower priority - performed within 3 months, and elective - could be postponed for > 3 months [25]. Table 2 provides a modified version of that provided in the ESC guidelines [25].
Table 2: Categorization and suggested time course for completion of cardiac procedures during the SARS-CoV-2 pandemic.
|
Cardiac Disease Category |
Emergent |
Urgent (within days to weeks) |
Time-sensitive (within 3 months) |
Elective (may postpone beyond 3 months) |
|
Ischemic heart disease |
-Revascularization for STEMI or NSTE-ACS (high-risk patients) -Revascularization in those with cardiogenic shock |
-Revascularization in left main disease, unstable angina, NTE-ACS (intermediate-risk patients), class IV angina symptoms -Revascularization in those with decompensated heart failure |
-Revascularization of proximal LAD lesions, class III angina symptoms |
-Revascularization for class II angina symptoms |
|
Valvular heart disease |
- Repair/replacement in those with cardiogenic shock due to valvular dysfunction |
-Decompensated AS -Hemodynamically unstable valvular regurgitation from ischemia or endocarditis -Endocarditis with high embolic risk |
-Severe AS (AVA < 0.6 cm 2 , mean gradient > 60 mmHg, symptoms with minimal exertion) -Symptomatic low-flow low-gradient AS (AVA < 1.0 cm 2 , mean gradient > 40 mmHg in patients with EF < 50%) -MR in patients with associated heart failure unresponsive to medical management |
-AS in those with AVA < 1.0 cm 2 +/- mean gradient > 40 mmHg -MR in patients with associated heart failure that is stable |
|
Heart failure |
- Mechanical circulatory support for cardiogenic shock |
-Heart transplant |
-LVAD |
|
|
Others |
- Cardiac tamponade - Type A aortic dissection or type B with evidence of end-organ injury - Ruptured TAAA - Traumatic injury resulting in hemodynamic compromise |
-Symptomatic left atrial myxoma -Contained rupture of TAAA in a hemodynamically stable patient -Rapidly expanding TAAA or excessively large (> 6.0-6.5 cm) |
-Biopsies |
-LAA occlusion -ASD/PFO closure -Interventions for hypertrophic cardiomyopathy -Stable TAAA |
Note: AS: Aortic stenosis; ASD: Anterior septal defect; AVA: Aortic valve area; LAA: Left atrial appendage; LAD: Left anterior descending coronary artery; LVAD: Left ventricular assist device; MR: Mitral regurgitation; NSTE-ACS: Non-ST-segment elevation acute coronary syndrome; PFO: Patent foramen ovale; STEMI: ST-segment elevation myocardial infarction; TAAA: Thoracoabdominal aortic aneurysm
During this "ramping up" of elective surgical cases, patients were also required to undergo pre-operative testing for SARS-CoV-2. Those who returned with positive tests were managed as according to the recommendations put forth by the American Society of Anesthesiologists and the Anesthesia Patient Safety Foundation [26]. In the case of a positive test, this entailed waiting until symptoms resolved completely. Widespread pre-operative testing was also recommended by most guidelines followed in other countries, though some recommended more extensive testing such as computed tomography for especially high-risk groups [27]. Difficulty did arise when patients who could not easily travel to a facility where testing could be administered. In most cases, the institution was able to coordinate the testing. Nevertheless, there were some circumstances where patients were managed as "SARS-CoV-2 unknown" and tested during their hospital admission. However, all recommendations did involve proceeding to surgery without testing for cases that needed to be completed within 24 hours, with testing eventually to occur later in their hospital admission (sometimes intra-operatively) [27]. This amongst other factors further complicated our surgical patients' post-operative care pathway and resource allocation.
Resource Management
An arduous period at our institution arrived after several patients who had tested negative for SARS-CoV-2 pre-operatively acquired the virus in the peri-operative period. At this same time, human resources were also strained as multiple staff members were required to quarantine due to potential exposures. This raised a number of questions regarding resource management. As many SARS-CoV-2 patients in our facility who required extracorporeal membrane oxygenation (ECMO) were housed in the cardiothoracic ICU, was it safe to bring patients who underwent elective procedures into the same unit as those with severe, active infections? Was there a better unit to house these patients in order to minimize their exposure to SARS-CoV-2? Was it appropriate to potentially use vital resources (nursing, ventilators, ECMO, blood products, personal protective equipment (PPE) etc.) for these patients and potentially deprive a patient with SARS-CoV-2? These questions were answered differently depending on the institution involved. At our institution (as at many others), patients with active SARS-CoV-2 infections were generally housed in isolated units to minimize exposure to other patients. Other institutions utilized central "hubs" where the majority of cardiac surgery took place, with outlying facilities serving as referral bases [24,28]. However, no organized system for directing time-sensitive cases was developed in our region, perhaps due to the relatively low-burden of cases compared to some areas of Europe where these systems were utilized. Ultimately, we had to defer elective cases further into the future than originally planned and divert some emergent/urgent cases that would have ultimately come to our institution. This occurred until the SARS-CoV-2 burden in the cardiothoracic ICU and facility as a whole subsided to a safe enough level to slowly begin doing elective cases again.
Moving forward, there are a several areas we could improve to prevent similar small "outbreaks" such as the one that occurred at our institution. While it may be convenient to have both SARS-CoV-2 patients on ECMO and elective cardiac surgical patients housed within the same unit, it may expose those who are not infected and place them at undue risk. During periods of high SARS-CoV-2 burden in which many patients may require ECMO, it would likely be beneficial to isolate these patients from the general and cardiac surgical patient populations. Likewise, more frequent cardiothoracic ICU staff testing (especially if displaying signs and symptoms of SARS-CoV-2 infection) would likely be prudent to minimize small outbreaks within facilities. Finally, continued staff and visitor screening and education is vital to promoting habits that prevent spread of infection and proper use of PPE.
Impact on Hospital Staff
Vital to resource management is the matter of human resources. Staff responsible for caring for large numbers of SARS-CoV-2 patients have been stretched thin over the course of the pandemic. This naturally has detrimental effects on one's psychological well-being. One cross-sectional study of residents and fellows at a major U.S. institution found that trainees exposed to SARS-CoV-2-infected patients had significantly higher degrees of stress and rates of burnouts compared to their peers who were not exposed [29]. While little data has been collected on this phenomenon, it is likely that stress, anxiety, and burnout in a group already prone to these maladies has been amplified during the pandemic. It is important that healthcare systems recognize and take appropriate steps to minimize the long-term sequelae of stress and burnout. This can be done by ensuring appropriate access to PPE, recruitment of additional staff to limit clinical duties, curtailing (or recruiting staff to assist with) administrative and other non-clinical responsibilities and providing easy access to wellness and psychiatric resources.
Conclusion
The crisis caused by SARS-CoV-2 continues to grip a considerable amount of the world healthcare apparatus. However, there are many valuable lessons provided by the pandemic that will help guide strategies to safely and effectively care for surgical patients during the current and future pandemics. The cardiac surgical patient population was quickly identified as a particularly high-risk group that would require a massive level of valuable resource allocation and concrete workflow adjustment. Guidelines from medical societies all over the world were quickly put into place to maximize the provision of critical care resources to patients suffering from severe SARS-CoV-2 infections, while also protecting and addressing those patients with serious and time-sensitive cardiovascular illnesses in need of surgical intervention. We are still discerning the long-term cardiovascular, pulmonary, and other systemic effects of SARS-CoV-2. It is possible that we will encounter these sequelae over the coming months and years as cardiac surgical case volumes increase. In the meantime, healthcare facilities worldwideare well-served continuing to use multi-disciplinary approaches to address the cardiac surgical workload in a manner that is safe for both patients and providers, and effectual in the preservation of scarce resources.
Conflict of Interests
The author reports no conflict of interests.
Funding
This work was supported by the Department of Anesthesiology and Pain Medicine, Department of Surgery and Department of Internal Medicine of University of California Davis Health System and NIH grant UL1 TR000002 of the University of California Davis Health.
References
- WHO Coronavirus Disease (COVID-19) Dashboard. World Health Organization. Retrieved November 11, 2020, from https://covid19.who.int/.
- Sanville B, Corbett R, Pidcock W et al. A Community-transmitted Case of Severe Acute Respiratory Distress Syndrome (SARS) Due to SARS-CoV-2 in the United States. Clin Infect Dis. 2020 Mar 30;ciaa347.doi: 10.1093/cid/ciaa347.
- Bonow RO, Fonarow GC, O’Gara PT et al. Association of Coronavirus Disease 2019 (COVID-19) With Myocardial Injury and Mortality. JAMA Cardiol. 2020;5(7):751-753. doi:10.1001/jamacardio.2020.1105.
- Yang J, Zheng Y, Gou X et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020 May;94:91-95.doi: 10.1016/j.ijid.2020.03.017.
- George I, Salna M, Kobsa S et al. The Rapid Transformation of Cardiac Surgery Practice in the Coronavirus Disease 2019 (COVID-19) Pandemic: Insights and Clinical Strategies From a Center at the Epicenter. Ann Thorac Surg. 2020 Oct;110(4):1108-1118.doi: 10.1016/j.athoracsur.2020.04.012.
- Shi S, Qin M, Shen B et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802-810. doi:10.1001/jamacardio.2020.0950.
- Guo T, Fan Y, Chen M et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):811-818. doi:10.1001/jamacardio.2020.1017.
- Zheng Y, Ma Y, Zhang J, and Xie X. COVID-19 and the cardiovascular system. Nature Reviews Cardiology volume 17, pages259–260(2020).
- Bilaloglu S, Aphinyanaphongs Y, Jones S et al. Thrombosis in Hospitalized Patients With COVID-19 in a New York City Health System. JAMA. 2020;324(8):799-801. doi:10.1001/jama.2020.13372.
- Connors JM and Levy JH. COVID-19 and its implications for thrombosis and anticoagulation.Blood (2020) 135(23): 2033–2040.https://doi.org/10.1182/blood.2020006000.
- Lemos ACB, do Espírito Santo DA, Salvetti MC et al. Therapeutic versus prophylactic anticoagulation for severe COVID-19: A randomized phase II clinical trial (HESACOVID). Thromb Res. 2020 Sep 21;196:359-366.doi: 10.1016/j.thromres.2020.09.026.
- Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497-506.doi: 10.1016/S0140-6736(20)30183-5. Epub 2020 Jan 24.
- Meng L, Qiu H, Wan L et al. Intubation and Ventilation amid the COVID-19 Outbreak: Wuhan’s Experience. Anesthesiology June 2020, Vol. 132, 1317–1332.https://doi.org/10.1097/ALN.0000000000003296.
- Lei S, Jiang F, Su W et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020 Apr 5;21:100331.doi: 10.1016/j.eclinm.2020.100331. eCollection 2020 Apr.
- Barkhordari K, Khajavi MR, Bagheri J et al. Early respiratory outcomes following cardiac surgery in patients with COVID-19. J Card Surg. 2020 Oct;35(10):2479-2485.doi: 10.1111/jocs.14915. Epub 2020 Aug 13.
- COVIDSurg Collaborative. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020 Jul 4;396(10243):27-38.doi: 10.1016/S0140-6736(20)31182-X. Epub 2020 May 29.
- Levy JH and Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 2003 Feb;75(2):S715-20.doi: 10.1016/s0003-4975(02)04701-x.
- Berrizbeitia LD, Tessler S, Jacobowitz IJ, Kaplan P, Budzilowicz L, and Cunningham JN. Effect of sternotomy and coronary bypass surgery on postoperative pulmonary mechanics. Comparison of internal mammary and saphenous vein bypass grafts. Chest. 1989 Oct;96(4):873-6. doi: 10.1378/chest.96.4.873.
- Mathis MR, Duggal NM, Likosky DS et al. Intraoperative Mechanical Ventilation and Postoperative Pulmonary Complications after Cardiac Surgery. Anesthesiology November 2019, Vol. 131, 1046–1062.https://doi.org/10.1097/ALN.0000000000002909
- Ad N, Luc JGY, Nguyen TC, and COVID-19 North American Cardiac Surgery Survey Working Group. Cardiac surgery in North America and coronavirus disease 2019 (COVID-19): Regional variability in burden and impact. J Thorac Cardiovasc Surg. 2020 Jul 2;S0022-5223(20)31983-8.doi: 10.1016/j.jtcvs.2020.06.077.
- Gaudino M, Chikwe J, Hameed I, Robinson NB, Fremes SE, and Ruel M. Response of Cardiac Surgery Units to COVID-19: An Internationally-Based Quantitative Survey. Circulation. 2020 Jul 21;142(3):300-302.doi: 10.1161/CIRCULATIONAHA.120.047865.Epub 2020 May 11.
- Haft JW, Atluri P, Ailawadi G et al. Adult cardiac surgery during the COVID-19 pandemic: A tiered patient triage guidance statement. J Thorac Cardiovasc Surg. 2020 Aug;160(2):452-455.doi: 10.1016/j.jtcvs.2020.04.011. Epub 2020 Apr 16.
- Hassan A, Arora RC, Lother SA et al. Ramping Up the Delivery of Cardiac Surgery During the COVID-19 Pandemic: A Guidance Statement From the Canadian Society of Cardiac Surgeons. Can J Cardiol. 2020 Jul;36(7):1139-1143.doi: 10.1016/j.cjca.2020.04.030. Epub 2020 Apr 29.
- Harky A, Harrington D, Nawaytou O et al. COVID-19 and cardiac surgery: The perspective from United Kingdom. J Card Surg. 2020 Sep 27;10.1111/jocs.15039.doi: 10.1111/jocs.15039.
- ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. European Society of Cardiology. Retrieved November 11, 2020, fromhttps://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance.
- ASA and APSF Joint Statement on Perioperative Testing for the COVID-19 Virus. American Society of Anesthesiologists and the Anesthesia Patient Safety Foundation. Retrieved November 11, 2020, from https://www.apsf.org/news-updates/asa-and-apsf-joint-statement-on-perioperative-testing-for-the-covid-19-virus/.
- Kovoor JG, Tivey DR, Williamson P et al. Screening and testing for COVID-19 before surgery. ANZ J Surg. 2020 Aug 7;10.1111/ans.16260.doi: 10.1111/ans.16260.
- Bonalumi G, Giambuzzi I, Barbone A et al. A call to action becomes practice: cardiac and vascular surgery during the COVID-19 pandemic based on the Lombardy emergency guidelines. Eur J Cardiothorac Surg. 2020 Aug 1;58(2):319-327. doi: 10.1093/ejcts/ezaa204.
- Kannampallil TG, Goss CW, Evanoff BA, Strickland JR, McAlister RP, and Duncan J. Exposure to COVID-19 patients increases physician trainee stress and burnout. PLoS ONE 15(8): e0237301. https://doi.org/10.1371/journal.pone.0237301.
Table of Contents
- Abstract
- Keywords
- Introduction
- The Effects of SARS-CoV-2 on the Cardiovascular and Pulmonary Systems
- Outcomes of Patients Undergoing Cardiac Surgery with SARS-CoV-2
- Changes to Operating Room throughout during the Pandemic
- Resource Management
- Impact on Hospital Staff
- Conclusion
- Conflict of Interests
- Funding
- Figure 1
- Table 1
- Table 2
- References