Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/165
Perspective | Volume 9 | Issue 4 Open Access
A Brief Overview of the Neuropharmacology of Opioid Addiction
John R Grothusen, PhD1*, Julie A Blendy, PhD2,3 and Gordon A Barr, PhD4,5
1Staff Scientist, Department of Anesthesiology and Critical Care, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
2Professor, Department of Systems Pharmacology and Translational Therapeutics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
3Vice-Chair, Department of Systems Pharmacology and Translational Therapeutics, Translational Research Laboratories, 125 South 31st St., Philadelphia, PA, USA
4Professor Emeritus, Department of Anesthesiology and Critical Care, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
5Department of Anesthesiology and Critical Care Medicine, Children's Hospital of Philadelphia, Philadelphia, PA, USA
John R Grothusen, PhD, Department of Anesthesiology and Critical Care, Perelman School of Medicine at the University of Pennsylvania, 337 John Morgan building, 3620 Hamilton Walk, Philadelphia, PA 19104, USA, E-mail: John.Grothusen@pennmedicine.upenn.eduEditor: Shuanglin Hao, MD, PhD, University of Miami Miller School of Medicine, United States of America, E-mail: shao@miami.edu
Received: October 20, 2022 | Accepted: November 29, 2022 | Published: December 05, 2022
Citation: Grothusen JR, Blendy JA, Barr GA. A Brief Overview of the Neuropharmacology of Opioid Addiction. Transl Perioper & Pain Med 2022; 9(4):491-496
Abstract
The world is in the midst of an opioid crisis. Nearly 92,000 persons in the U.S. alone died from illicit drugs and prescription opioids in 2020 [1]. This number does not include the countless other individuals who die as a result of the violent crime that accompanies the illicit drug trade. To address this crisis, we need to appreciate aspects of drug addiction. The goal of this brief review is to highlight some major facets of addiction neurobiology, focused on opioids, to provide a basic understanding of the research and terminology encountered in more detailed in-depth articles and discussions on addiction.
Main Text
Physical and psychological dependence versus addiction
Physical dependence is defined by physical/somatic changes that occur when a drug is withdrawn and occurs as the body adapts to a drug, by metabolism or receptor changes that occur naturally with long term use. It is often accompanied by tolerance which is when more drug is required to achieve a desired effect, defined pharmacologically as a shift in the dose-response curve to the right.
Psychological dependence is the mental need and craving for a drug. It can occur even with drugs that have few physical withdrawal consequences, such as marijuana. "Craving" is part of psychological dependence, often in the absence of physical signs. Moreover, it can be associated with cues related to drug use (e.g. location, other users, situations) to serve as powerful stimuli to elicit continued psychological dependence and craving.
Addiction is characterized by compulsive drug use despite harmful consequences, which can include failure to meet work, social, or family obligations, crime and even death. The importance of positive or negative reinforcing properties of drugs inestablishing compulsive drug use is a much-debated topic in the field. The intense pleasure of a drug is presumed to reflect a positive rewarding processes, and the negative state associated with the drug wearing off or abstinence. The withdrawal symptoms associated with abstinence can be physical, psychological, or both. For opioid dependence somatic signs can be quite severe on cessation of drug use, and individuals may return to using the drug as a way to relieve these aversive side effects. But addiction goes beyond mere physical and psychological dependence, relapse can occur in the absence of acute withdrawal signs, and some have argued that all drug addictions have a psychological dependence component [2]. Physical withdrawal signs may contribute to the addiction process but not necessarily so.
Addiction and brain reward circuit
There are a wide variety of drugs that are addictive. Drugs, such as cocaine, methamphetamine, opioids, alcohol and nicotine, to name a few, differ widely in their effects, pharmacology and receptor binding. Despite varying mechanisms of action, all are capable of causing addiction, craving and withdrawal that can be extremely debilitating.
Multiple and redundant neural circuits are involved in the various aspects of addiction. However, most drugs of abuse modulate their effects via the mesolimbic dopamine system. These neurons are part of a well-defined pathway involved in reward processing. This pathway consists primarily of dopamine fibers arising in the ventral tegmental area (VTA) and projecting to the nucleus accumbens (NAc). GABAergic interneurons in the VTA maintain a tonic inhibition over dopaminergic neurons, whereas glutamatergic inputs from the prefrontal cortex serve to excite these neurons [3-5]. Depending on the specific drug and its direct receptor target, the primary site of action within this pathway may vary. For example, nicotine acts on nicotinic receptors (nAChR) which are present throughout the brain on dopamine, GABA and glutamate containing neurons [6], and the relative amounts of receptors and subunit specificity can vary from cell to cell. However, it is the activation of nAChR receptors specifically in the VTA that is thought to underlie the rewarding effects of nicotine. Similarly, much of the rewarding properties of opioids are mediated in the VTA. Activation of mu-opioid receptors on GABAergic neurons in the VTA results in a decrease in activity of these neurons, resulting in disinhibition of dopamine neurons. The subsequent increase of dopamine in the NAc is associated with drug reinforcement [7,8]. While most of the rewarding properties of both opioids and nicotine are mediated to a large extent by the VTA, this is in contrast to cocaine, which is thought to exert its rewarding effect by increasing extracellular levels of dopamine in the NAc by blocking dopamine transporters [5]. Although a simplification, for purposes of this brief perspective, it is believed that most classes of addictive drugs, share the common property of altering dopamine signaling within the mesolimbic pathway [9] (Figure 1).
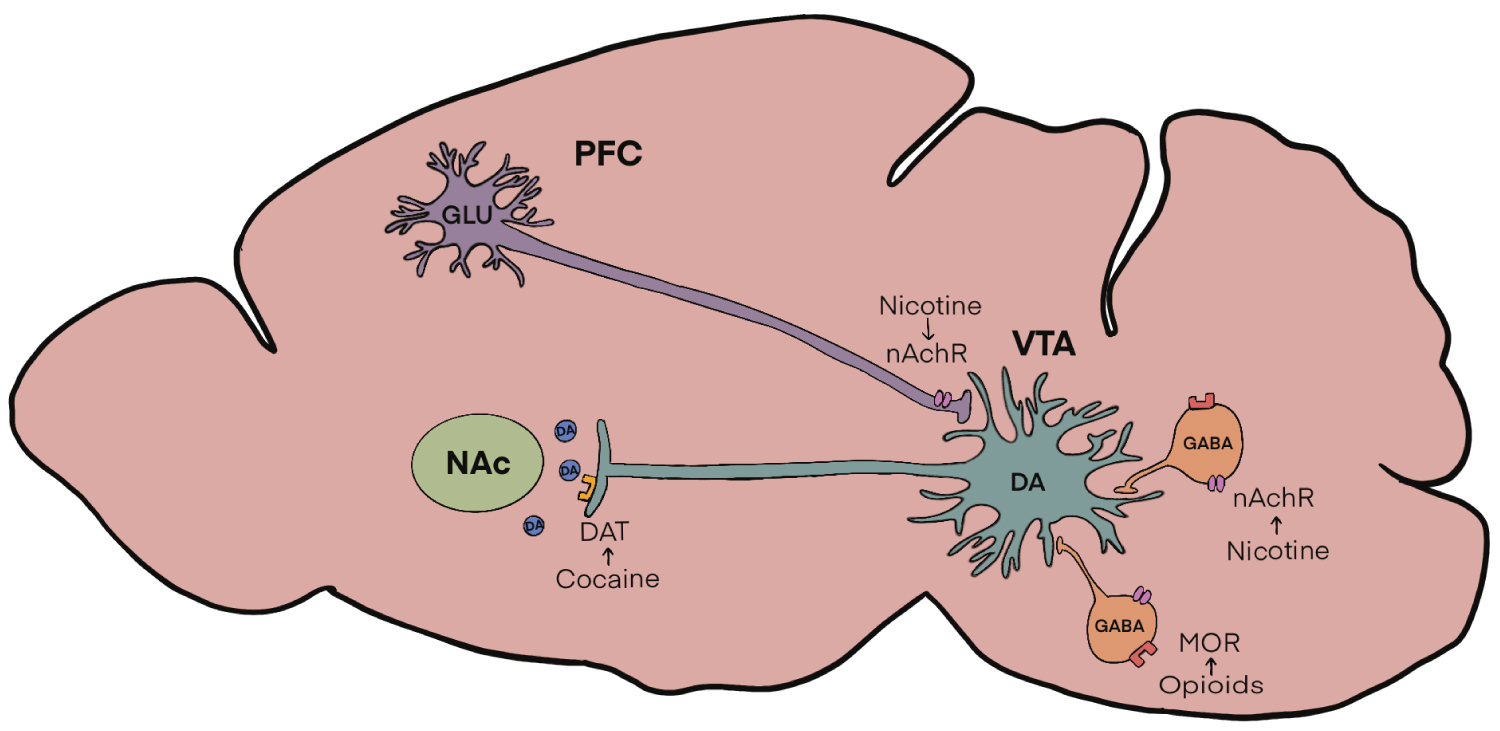
Figure 1: Figure 1: Mesolimbic Dopamine Circuitry. Dopamine (DA) cell bodies in the Ventral Tegmental Area (VTA) are modulated by GABAergic interneurons containing mu-opioid receptors (MOR) as well as neuronal nicotinic acetylcholine receptors (nAchR). AchRs are also located on glutamatergic inputs from the prefrontal cortex (PFC). All these inputs regulate the release of dopamine in the nucleus accumbens (NAc) which is then taken back up presynaptically by dopamine transporters (DAT).
Psychomotor stimulant and alcohol addiction
Two highly addictive drugs, cocaine and methamphetamine, bind to dopamine reuptake transporters, which normally clear synapses of released dopamine, thus increasing the amount of dopamine and its residency in the synapse. This results in a heightened response and feelings of euphoria. Alcohol use has been shown to increase dopamine release from the VTA and chronic use of alcohol has been posited to result in an imbalance of the normal VTA reward/aversion functioning [10]. There has been a large amount of research on the VTA and its projections to the nucleus accumbens and other components of reward circuits like the frontal cortex and the amygdala. A full discussion of this is beyond the scope of this brief paper, but it is important to mention the "dopamine hypothesis" of drug addiction to give a perspective on how drugs with very different actions and effects, such as opioids, methamphetamine, cocaine and alcohol to name a few, can all share a common neurobiological mechanism of addiction.
Opioid addiction
Opioids are the most potent analgesics for severe pain, and as such have a legitimate and very important medical use. Technically the term opioid refers to any agent that binds to opioid receptors, including endogenous opioid peptides, and opium alkaloids such asmorphine, codeine, heroin and fentanyl. In contrast, the term opiate refers to alkaloids found in opium which is extracted from the poppy flower, such as all natural and semi-synthetic derivatives of morphine. For purposes of this review the term opioids will be used in reference to both opioids and opiates. Opioid addiction, along with the many personal and societal issues that accompany drug addiction in general, poses additional issues that must be considered. The first issue is that many people became addicted to opioids due to medical treatment with prescription opioid painkiller drugs. They became part of the opioid crisis not from recreational use, but from a legitimate medical use. The second issue is that opioids have a very dangerous side effect of respiratory depression. Moreover, opioids can synergize with other drugs and alcohol to increase risk of respiratory depression which is the main cause of opioid overdose death, which at the present time is a leading cause of death among young adults.
Opioids act through endogenous receptors. Opioid receptors are a group of transmembrane inhibitory G protein-coupled receptors with endogenous opioid peptides as their natural ligands. The opioid receptors and their endogenous ligands are involved in mediating many bodily functions, including sensory perception. The endogenous opioid system consists of three families of opioid peptides, the beta-endorphins, enkephalins, and dynorphins, that interact with four subtypes of opioid receptors, the mu (MOR), delta (DOR), kappa (KOR), and N/OFQ (NOP) opioid receptor subtypes. Studies on drug abuse mechanism have focused on MOR since there are no currently FDA approved DOR or NOP selective agonists to treat addiction and although KOR antagonists may have potential as therapeutics, their use clinically has been hampered by adverse side effects.
Stimulation of MOR with various opioids results in feelings of euphoria, which is a significant reason for illicit use of opioids. This effect is in keeping with the natural endogenous agonists of the MOR, the beta-endorphins, which are sometimes referred to as the "feel good hormones" and have been associated with the positive emotional reward of human experiences such as eating, human interactions, laughing, exercising and sexual activity.
The reinforcing, rewarding and therefore addictive properties of opioids appear to be mediated primarily by MOR opioid receptors on inhibitory GABAergic interneurons in the VTA [11]. Activating these receptors disinhibits the dopamine containing neurons, which then results in increased dopamine release from the VTA to the nucleus accumbens.
Of interest, not all individuals who use or even abuse drugs go on to develop an addiction. Genetic factors account for 50-60% of disease risk for alcohol and smoking, with an even higher inherited risk of up to 80% for opioid and cocaine dependence [12,13]. Specifically, a common genetic variant in the gene encoding the MOR alters MOR protein levels and binding affinity and this variant has been associated with an altered vulnerability to opioid addiction [14,15], and a decreased response to opioid-induced analgesia [16,17]. Thus, while complex associations related to receptor pharmacology and neural circuits are important, genetics may be used to identify the causes of individual differences in response to commonly abused drugs or to inform a more precise preventive care or treatment for addiction.
In contrast to the pharmacology of MOR receptors, opioid binding to KOR receptors appears to decrease dopamine release from the VTA to the nucleus accumbens [9]. The KOR in the VTA appears to bepart of an anti-reward system that leads to dysphoria and might possibly function as a control to limit the motivational properties of rewarding stimuli, including drugs of abuse [18]. Conversely, increased expression of KOR following prolonged exposure to drugs of abuse may contribute to aversive symptoms of acute withdrawal, and depressive symptoms during long phases of abstinence.
Full vesus partial opioid agonists and biased agonists
Two very important topics in opioid pharmacology are the concepts of full and partial agonists, and biased agonists. Opioid receptors are transmembrane receptors that undergo intricate conformational changes when a ligand binds to the extracellular binding site. Depending on how a specific ligand binds to the receptor, it may cause a full maximal intracellular response, or only a partial intracellular response. Drugs such as morphine, oxycodone, heroin and fentanyl are considered full MOR agonists, causing maximum intracellular responses and bodily effects that include analgesia, respiratory depression and addiction.
When a ligand binds to the surface receptor it causes a number of intracellular actions to occur. Two intracellular protein mediated pathways are activated upon binding of an appropriate ligand to the extracellular domain of the receptor. One pathway is the G protein (GPCR) pathway, where subunits dissociate from the receptor and the net result is an inhibition of the enzyme adenylate cyclase. This causes a reduction in the intracellular concentration of cyclic-adenosine monophosphate (cAMP) an important mediator of intracellular signaling. The second pathway activated by ligand binding to an opioid receptor involves proteins of the β-arrestin pathway. These β-arrestin proteins are scaffold proteins and are involved in a myriad of intracellular actions too numerous to detail here [19]. Cell culture based assays have been developed to measure the activities of the G protein and the β-arrestin pathways independently. Based on results of these assays the concept of biased signaling has been posited. Initially the concept arose that opioids that preferentially activated the GPCR pathway may be efficacious analgesics with less adverse effects such as tolerance, addiction and respiratory depression which were attributed to activation of the β-arrestin pathways [20]. It is important to point out that the concept that biased ligands that activate the GPCR pathway preferentially provide analgesia devoid of adverse actions attributed to β-arrestin pathway activation is an oversimplification. In fact, the concept of biased ligands has been questioned. Some researchers believe that the appearance of bias may be a property of partial opioid agonists in general [21].
The details of assays for bias and partial agonists is beyond the scope of this brief overview, however the hypothesis that biased signaling might distinguish analgesia and adverse effects has led to a resurgence of interest in synthesizing and re-discovering opioids with analgesic efficacy and reduced adverse effects. The first opioid drug specifically designed to be GPCR biased has been approved by the FDA and marketed by Trevena Inc. This drug, Olinvyk (Oliceridine) has been shown to have equal analgesic potency to morphine and a potentially better safety profile.However, in spite of a high bias ratio (GPCR activity/β-arrestin activity) concerns about possible abuse and addiction liability have limited its current approved use to intravenous post-operative pain relief only under strict medical supervision.
Treatment of opioid addiction
Addiction recovery medicine has taken on an increasingly important role in addiction treatment. There are multiple treatment modalities that are currently in use to treat patients with opioid addiction. These can be divided into two main categories, psychology based interventions such as 12 step programs, and medication for opioid use disorder (MOUD) treatments. The most popular peer support groups use a similar approach to groups like Alcoholics Anonymous which follow 12 steps towards addressing spiritual development and personal growth with a sponsor and attending 12-step community events. This type of self-help peer support approach is the most utilized treatment for patients with OUD, as opposed to MOUD which uses medically supervised use of drugs such as methadone or buprenorphine as replacements for the abused opioid or naltrexone to block the rewarding effects of the abused opioid [22]. Naltrexone is a long acting MOR antagonist that blocks the euphoria if the patient takes an opioid. Naltrexone can be administered as a daily oral medication (i.e. ReVia, Depade) or as a monthly injection (i.e. Vivitrol) to block the effects of opioids. The use of naltrexone therapy requires that the patient first undergo withdrawal or detoxification from opioids before beginning this treatment approach. The MOUD approach, using buprenorphine or methadone, has been associated with reductions in overdose and serious opioid-related acute care use compared with other, non-MOUD treatments in a large retrospective study [22]. Unfortunately MOUD is underused due to multiple factors that include higher expense compared to peer group self-help programs, and the stigma among many that MOUD is just substituting one addictive drug for another.
Addiction treatment requires a multifaceted approach, and in the case of opioid addiction, often uses replacement therapy. This therapy involves replacing the abused opioid with another, less euphoric, drug, with more favorable pharmacokinetics, that hopefully reduces the craving and drive for the abused drug. Replacement therapy also is designed to reduce opioid withdrawal symptoms in the hope of preventing relapse. The two most used replacement drugs to treat opioid addiction, or opioid use disorder, are methadone and buprenorphine.
Methadone is a full MOR agonist that has been used for decades and is administered by supervised oral dosing inlicensed opioid treatment programs or methadone clinics. The ability to administer methadone orally is a major reason it is used in opioid replacement therapy. Since oral dosing slows down absorption and entry into the brain it doesn't produce the sought-after euphoria in opioid-dependent individuals. Moreover, unlike other abused opioids, its long half-life (~24+ hours) allows for practical use in recovering addicts. The controlled and supervised chronic dosing is meant to reduce cravings and withdrawal symptoms, thus allowing the compliant and motivated individual to transition away from the much more dangerous use of street drugs. Methadone does produce physical dependence and acute withdrawal symptoms just like the opioid drugs it is meant to replace.
Buprenorphine, a partial MOR agonist and a KOR receptor antagonist, is distinct from methadone and most of the highly addictive prescribed and illicit opioids. When used correctly, buprenorphine does not produce the craved euphoria effect in opioid-dependent patients. Its binding to MOR is slow and since it is only a partial MOR agonist there is a more limited ability to stimulate reward circuits. The search for new and novel mixed partial opioid agonists may be a viable strategy for replacement therapy since the current strategy of opioid replacement using methadone or buprenorphine, while effective treatments, both induce significant respiratory depression and have abuse potential themselves. Methadone is a drug of abuse and subject to diversion when it is administered by routes other than oral [23]. Buprenorphine, as well is prone to abuse and diversion for non-medical use [24]. Naloxone, a full MOR antagonist, is added to buprenorphine (Suboxone) as a means to block euphoric effects and prevent misuse and diversion, such as intravenous administration.
One drug in particular that deserves to be considered for replacement therapy as a potential alternative to methadone or buprenorphine is dezocine, which is an opioid analgesic that was produced by American Home Products (Wyeth) and approved by the USA FDA in 1986 [25]. It is a more potent analgesic than morphine [26], but displays much reduced respiratory depression and no obvious addictive properties [25]. It was used as a post-operative analgesic until its distribution was discontinued in the USA in 2011, but is used extensively as a perioperative analgesic drug in China [27]. Dezocine is not a scheduled medication as classified by the World Health Organization or the FDA, and no cases addiction has been reported in the literature [25].
Dezocine is a partial MOR and a partial KOR agonist [25,28,29]. Because it is a partial MOR agonist, this may explain its greatly reduced respiratory depression effects and apparent lack of addictive properties. Being a partial MOR agonist like buprenorphine (and unlike the full MOR agonist methadone), dezocine is also a biased MOR ligand, displaying no significant activation of the β-arrestin pathway using a cell culture based Tango assay [30-32]. Buprenorphine is a non-biased ligand and displayed significant activation of the β-arrestin pathway when assayed under the same conditions and concentrations as dezocine [30]. β-arrestin activation by MOR agonists has been associated with serious adverse effects of MOR activation such as respiratory depression and addiction [20]. Dezocine alleviates morphine withdrawal symptoms in an animal model of opioid use disorder [33]. A medication such as dezocine may offer a better alternative than methadone or buprenorphine replacement therapy for treatment of opioid addiction because of its superior qualities that include lack of reported abuse and addiction and reduced lethality.
Conclusion
Addiction, especially opioid addiction, is a complex disease that affects many people and has ruined far too many lives. This brief overview was intended to provide a basic understanding of some of the major neuropharmacological mechanisms that are involved. Opioids are still the most effective analgesic drugs for moderate to severe pain, and as such, will be an integral part of pain management for the foreseeable future. The need for new and re-discovered opioid medications that provide effective analgesia, but have reduced adverse effects such as respiratory depression and a lower risk for addiction becomes more critical with each passing day as the world continues to confront the 'opioid crisis' which was created in large part from the over-use of powerful and addicting opioid drugs by many well-intentioned pain management professionals.
Funding
Dr. Blendy acknowledges support from NIDA grant DA044743.
Acknowledgement
The authors wish to acknowledge Emma Tyner for assistance with artwork for the figure.
References
- NIDA. Overdose death rates. https://nida.nih.gov/drug-topics/trends-statistics/overdose-death-rates. 2022
- Sripada C. Impaired control in addiction involves cognitive distortions and unreliable self-control, not compulsive desires and overwhelmed self-control. Behav.BrainRes. 2022;418:113639
- Samathanam G, Duffy P, Kalivas PW, White SR. A comparison of 5-hydroxytryptophan effects on rat lumbar spinal cord serotonin release and monosynaptic response amplitude. Brain Res. 1989;501:179-182
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci. 1992;12:483-488
- Taber MT, Das S, Fibiger HC. Cortical regulation of subcortical dopamine release: Mediation via the ventral tegmental area. J. Neurochem. 1995;65:1407-1410
- Keath JR, Iacoviello MP, Barrett LE, Mansvelder HD, McGehee DS. Differential modulation by nicotine of substantia nigra versus ventral tegmental area dopamine neurons. J. Neurophys. 2007;98:3388-3396
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274-5278
- Shoaib M, Spanagel R, Stohr T, Shippenberg TS. Strain differences in the rewarding and dopamine-releasing effects of morphine in rats. Psychopharmacology (Berl). 1995;117:240-247
- Langlois LD, Nugent FS. Opiates and plasticity in the ventral tegmental area. ACS Chem.Neurosci. 2017;8:1830-1838
- You C, Vandegrift B, Brodie MS. Ethanol actions on the ventral tegmental area: Novel potential targets on reward pathway neurons. Psychopharmacology (Berl). 2018;235:1711-1726
- Galaj E, Han X, Shen H, Jordan CJ, He Y, Humburg B, et al. Dissecting the role of gaba neurons in the vta versus snr in opioid reward. J. Neurosci. 2020;40:8853-8869
- Agrawal A, Lynskey MT. Are there genetic influences on addiction: Evidence from family, adoption and twin studies. Addiction. 2008;103:1069-1081
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: Uncovering the genes. Nat. Rev. Genet. 2005;6:521-532
- van den Wildenberg E, Wiers RW, Dessers J, Janssen RG, Lambrichs EH, Smeets HJ, et al. A functional polymorphism of the mu-opioid receptor gene (oprm1) influences cue-induced craving for alcohol in male heavy drinkers. Alcohol.:Clin. Exp. Res. 2007;31:1-10
- Drakenberg K, Nikoshkov A, Horvath MC, Fagergren P, Gharibyan A, Saarelainen K, et al. Mu opioid receptor a118g polymorphism in association with striatal opioid neuropeptide gene expression in heroin abusers. Proc Natl Acad Sci USA. 2006;103:7883-7888
- Chou WY, Yang LC, Lu HF, Ko JY, Wang CH, Lin SH, et al. Association of mu-opioid receptor gene polymorphism (a118g) with variations in morphine consumption for analgesia after total knee arthroplasty. Acta Anaesthesiol. Scand. 2006;50:787-792
- Sia AT, Lim Y, Lim EC, Goh RW, Law HY, Landau R, et al. A118g single nucleotide polymorphism of human mu-opioid receptor gene influences pain perception and patient-controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology. 2008;109:520-526
- Lalanne L, Ayranci G, Kieffer BL, Lutz PE. The kappa opioid receptor: From addiction to depression, and back. Front. Psychiatry. 2014;5:170
- Ahn S, Shenoy SK, Luttrell LM, Lefkowitz RJ. Snapshot: Beta-arrestin functions. Cell. 2020;182:1362-1362 e1361
- Porter-Stransky KA, Weinshenker D. Arresting the development of addiction: The role of beta-arrestin 2 in drug abuse. J Pharmacol Exp Ther. 2017;361:341-348
- Azevedo Neto J, Costanzini A, De Giorgio R, Lambert DG, Ruzza C, Calo G. Biased versus partial agonism in the search for safer opioid analgesics. Molecules. 2020;25
- Wakeman SE, Larochelle MR, Ameli O, Chaisson CE, McPheeters JT, Crown WH, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA network open. 2020;3:e1920622
- Lugoboni F, Zamboni L, Cibin M, Tamburin S, Gruppo Inter SdCS. Intravenous misuse of methadone, buprenorphine and buprenorphine-naloxone in patients under opioid maintenance treatment: A cross-sectional multicentre study. Eur. Addict. Res. 2019;25:10-19
- Lavonas EJ, Severtson SG, Martinez EM, Bucher-Bartelson B, Le Lait MC, Green JL, et al. Abuse and diversion of buprenorphine sublingual tablets and film. J. Subst. Abuse Treat. 2014;47:27-34
- Childers WE, Abou-Gharbia MA. "I'll be back": The resurrection of dezocine. ACS Med.Chem. Lett. 2021;12:961-968
- Malis JL, Rosenthale ME, Gluckman MI. Animal pharmacology of wy-16,225, a new analgesic agent. J Pharmacol Exp Ther. 1975;194:488-498
- Shi H, Chen X, Liu X, Zhu H, Yu F, Ung COL, et al. National drug utilization trend of analgesics in china: An analysis of procurement data at 793 public hospitals from 2013 to 2018. J. Pharm.Policy Pract. 2021;14:45
- Wang YH, Chai JR, Xu XJ, Ye RF, Zan GY, Liu GY, et al. Pharmacological characterization of dezocine, a potent analgesic acting as a kappa partial agonist and mu partial agonist. Sci Rep. 2018;8:14087
- Ye RR, Jiang S, Xu X, Lu Y, Wang YJ, Liu JG. Dezocine as a potent analgesic: Overview of its pharmacological characterization. Acta Pharmacologica Sinica. 2021
- Grothusen J LW, Xi J, Zanni G, Barr G, Liu R. Dezocine is a biased ligand without significant beta arrestin activation of the mu opioid receptor. Transl Periop & Pain Med. 2022;9:424-429
- Barnea G, Strapps W, Herrada G, Berman Y, Ong J, Kloss B, et al. The genetic design of signaling cascades to record receptor activation. Proc Natl Acad Sci U S A. 2008;105:64-69
- Kroeze WK, Sassano MF, Huang XP, Lansu K, McCorvy JD, Giguere PM, et al. Presto-tango as an open-source resource for interrogation of the druggable human gpcrome. Nat Struct Mol Biol. 2015;22:362-369
- Wu FX, Babazada H, Gao H, Huang XP, Xi CH, Chen CH, et al. Dezocine alleviates morphine-induced dependence in rats. Anesth. Analg. 2019;128:1328-1335