Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/183
Review Article | Volume 11 | Issue 1 Open Access
Anesthesia Management For Pediatric Liver Transplantation: Narrative Review
Nedim Çekmen, MD and Ahmed Uslu, MD*
Department of Anaesthesia and Intensive Care, Başkent University Ankara Hospital, Ankara, Turkey
Ahmed Uslu, Department of Anaesthesia and Intensive Care, Anaesthesiology and Intensive Care Medicine, Başkent University Ankara Hospital, Ankara, Turkey, E-mail: ahmed.uslu@hotmail.comEditor: Renyu Liu, MD; PhD; Professor, Department of Anesthesiology and Critical Care, Perelman School of Medicine at the University of Pennsylvania, Center of Penn Global Health Scholar, 336 John Morgan building, 3620 Hamilton Walk, Philadelphia, PA 19104, USA, Fax: 2153495078; E-mail: RenYu.Liu@pennmedicine.upenn.edu
Received: December 07, 2023 | Accepted: February 07, 2024 | Published: February 07, 2024
Citation: Çekmen N, Uslu A. Anesthesia Management For Pediatric Liver Transplantation: Narrative Review. Transl Perioper Pain Med 2024; 11(1):574-591
Abstract
Pediatric liver transplantation (LT) is the decisive treatment for end-stage liver disease (ESLD). Indications for pediatric LT only due to ESLD can be applied to the causes and an underlying metabolic or genetic syndrome. Understanding the complex pathophysiology of ESLD and its complications in the affected systems is essential for proper anesthesia management in LT. LT is a dynamic process preoperative optimization is essential in these patients and requires a multidisciplinary team approach. Anesthesia of the pediatric patient undergoing LT is challenging, and therefore, anesthesiologists should focus on rapidly fluctuating physiology, hemodynamics, metabolic, and coagulation status in managing these patients' associated comorbidities. Anesthesia management in LT requires specialized management that requires adequate knowledge, attitude, multidisciplinary approach, and equipment. In our review, we aimed to present the perioperative and anesthesia management in pediatric LT patients.
Keywords
Pediatric patient, Liver transplantation, Anesthesiologists, Perioperative management, Multidisciplinary and specialized teams
Introduction
Objectives: (1) To emphasize the importance of preoperative evaluation in patients undergoing pediatric LT. (2) Review the importance of anesthesiologists in the rapidly changing physiology, hemodynamic, metabolic, and coagulation conditions in pediatric patients undergoing LT. (3) Discuss the anesthetic management in LT comprehensively according to its phases. (4) To highlight comprehensive intraoperative critical preventive methods, cardiovascular monitoring, drug use, fluid therapy, extubation time, immunosuppression, pain, nutritional management, and postoperative care. (5) To emphasize that anesthesia management in LT is a specialized management that requires adequate knowledge, attitude, multidisciplinary approach, and equipment.
Pediatric LT has been an effective and widely accepted treatment for ESLD in infants and children over the past two decades and is a highly complex surgical procedure [1]. Pediatric LT has many indications, the most common being biliary atresia, toxicities, and metabolic, genetic, and infectious diseases [2-4]. Identified indications and absolute contraindications for pediatric LT are given in Table 1. Our data shows that 306 patients underwent living donors, and the most common indications for LT are biliary atresia, biliary atresia where a Kasai procedure has failed glycogen store disorders, and genetic disorders. In our center, the mean age of the patients was 5.8, the Pediatric End-Stage Liver Disease (PELD) score was 12.3, they had many comorbidities, and one-year survival was 90.1%. ESLD requiring LT may have multisystem disorders that require significant anesthetic approaches. However, when an LT is indicated for a child to benefit from the transplant, all perioperative specialties related to the patient should discuss the patient's comorbidities and risks and form a consensus in a multidisciplinary approach [4-7].
Table 1: Preoperative assessment.
|
Interaction mode |
Interacting residues |
|
Polar |
THR97 |
|
Hydrophobic |
LEU89, ALA90, VAL93, CYS96, ILE100, PRO147, LEU166, TRP384, PHE388 |
|
Pi-pi |
TRP384, PHE387, PHE412, TYR416 |
|
Other |
ASP92, VAL93, THR97, LEU166, |
Preoperative Assessment
In pediatric patients with ESLD, symptoms and signs such as coagulopathy, ascites, and varicose veins are typical. However, such characteristic findings can sometimes be overlooked in patients with ESLD due to metabolic diseases or genetic syndromes. Proper anesthesia management in LT requires understanding the complex pathophysiology of ESLD and its complications in the affected cardiovascular, pulmonary, kidney, nervous, gastrointestinal, and hematological systems. The most important of these are cerebral edema, hepatic encephalopathy (HE), hepatorenal syndrome (HRS), pulmonary hypertension (PHT), and hepatopulmonary syndrome (HPS), and these complications lead to increased morbidity and mortality in the perioperative period [2-5]. LT anesthesia management is a complex procedure that requires a multidisciplinary team approach. This team includes a hepatologist, pediatrics transplant surgeon, pediatric anesthesiologist, intensivist, transplantation coordinator, specialty nurses, and other specialties consulted as indicated based on their underlying diagnosis (Pulmonology, Cardiology, Genetics, Nephrologist, etc.) and social care professionals (psychologist, dietitian, physiotherapist). Pediatric transplant anesthesiologists are physicians in pediatric LT who require sufficient knowledge and expertise in anesthetic aspects of ESLD and associated comorbidities. Pediatric transplant anesthesiologists should perform a comprehensive preoperative evaluation of patients indicated for LT and optimize their medical condition, physical examination, cardiopulmonary changes, metabolic disorders, difficulties with vascular access, coagulopathy and blood management, and blood products to prepare for potential challenges. Pediatric transplant anesthesiologists should determine the necessary preventive strategies during preoperative planning with a multidisciplinary approach and close communication with the surgeon and hepatologist [1,4-6].
ESLD should be considered a systemic disease that causes dysfunction in various organ systems. The presence of one or more organ dysfunction significantly increases the risk of death in these patients without LT [8]. Patients with ESLD may have a high cardiac output (CO) and low resistance systemic circulation. Patients with ESLD usually have hyperdynamic circulation with increased heart rate (HR), stroke volume (SV), CO, decreased systemic vascular resistance (SVR), and low blood pressure (BP). As a result of the hyperdynamic circulation, these patients have central hypovolemia and/or have poor hemodynamic tolerance in any concomitant cardiovascular pathology [9]. Low SVR may result from increased endogenous production or decreased hepatic clearance of vasodilators such as nitric oxide (NO), carbon monoxide (CO), endogenous cannabinoids, TNF-α, adrenomedullin, and hydrogen sulfide. In addition, there is an inflammatory response due to bacterial translocation, which usually causes splanchnic arterial vasodilation. In response to low SVR, Na + and water retention, which increase plasma volume, are the most important mechanisms. There is also increased venous capacitance where any portosystemic shunt exists [2,5,6,8,9].
Some diseases that cause ESLD, such as hemochromatosis, Wilson's disease, and amyloidosis, may contribute to cardiovascular dysfunction. Therefore, these patients should be treated with great care regarding hemodynamic instability. With any combination of organomegaly, ascites, or pleural effusions, patients with ESLD may develop significant complications due to various mechanical events, and these may worsen respiration by significantly reducing lung volume and, thus, functional residual capacity (FRC) [1-3,5,6].
In summary, the conditions to be considered in the preoperative evaluation are as follows:
• Cause and consequences of LT indication
• The child's general health, hemodynamic, metabolic, nutritional, consciousness, and allergy status, age, weight, height, and necessary scoring (American Society of Anesthesiologists (ASA), Mallampati, the pediatric end-stage liver disease = PELD, the model for end-stage liver disease sodium = MELD-Na + ) should be noted [2-4].
• Effects and consequences of ESLD (varices, portal hypertension, and other complications) [4,5].
• ESLD may present as one of the genetic and metabolic syndromes, particularly Alagille syndrome, and many of these, particularly cardiac and many other anomalies, may present difficulties for the anesthetist during LT [2,4-6].
• Kidney dysfunction is significant in children with ESLD, and its causes may be multifactorial. The most common causes are polycystic kidney disease due to polycystic liver disease (which may occur with primary oxaluria), HRS, prerenal azotemia, and acute tubular necrosis (ATN) [4-6].
• HPS or porto-pulmonary diseases can create an oxygen-dependent state due to hypoxemia. These children should always have thorough transthoracic echocardiography (TTE) before surgery to evaluate cardiac function and PHT grade. Cardiac catheterization may also be necessary in cases such as advanced PHT, valvular insufficiency, and additional congenital heart diseases (CHD). Pre-existing right-to-left shunts will raise the risk of systemic air embolism while performing venous anastomoses in the intraoperative period; thus, care should be taken and necessary precautions [4-6,8].
• The most critical causes of preoperative hypoxemia are intrapulmonary arteriovenous shunt, ventilation (V)-perfusion (Q) mismatch, reduced pulmonary diffusion capacity, restrictive lung disease caused by ascites, and raised intra-abdominal pressure. These reasons should be carefully considered in the intraoperative period, and necessary precautions should be taken, as they will shift the oxygen dissociation curve to the right and cause oxygenation impairment [5,6,8,9].
• Patients with arterio-venous shunts have raised venous saturations and decreased arterio-venous oxygen content difference. Therefore, these patients should not be ignored as they are predisposed to supraventricular tachycardias, valvular lesions, cardiomyopathies, and rarely biventricular failure [6,8,9].
• The existence of esophagus, stomach, rectal, or abdominal wall varicose veins and treatments should be considered. These patients are usually anemic before surgery due to bleeding varices and/or HRS [3-6].
• Conditions such as biliary atresia with Kasai Porto-enterostomy, varicose veins, shunts, and the presence of previous abdominal surgery should be questioned in detail in the preoperative period, as they will increase the amount of bleeding in the prehepatic phase and affect the time before the diseased liver is removed [2,4-6].
• The preoperative fasting period is essential in these patients, and special fluids should be started in terms of hypoglycemia or hyperglycemia according to metabolic/genetic diseases or encephalopathy status [10,11].
• It must be confirmed that the blood bank, hematology, and biochemistry laboratories are informed about the operation in the preoperative period [5-7,10,11].
• A plan should be made in advance for postoperative care, and necessary preparations should be made in the PICU [10,11].
ESLD should be considered a systemic disease that causes dysfunction in various organ systems. The presence of one or more organ dysfunction significantly increases the risk of death in these patients without LT. Laboratory tests and imaging methods that should be done according to the departments in preoperative patient preparation are standard [4-7,10,11]. The council evaluates the parameters in our center, and operational decisions are made (Table 2).
Table 2: Indications and contraindications for pediatric liver transplantation.
|
Indications |
|
|
Cholestatic disorders |
Metabolic disorders without cirrhosis |
|
Extra-hepatic biliary atresia |
Urea cycle disorders |
|
Intrahepatic biliary hypoplasia (Alagille disease) |
Crigler-Najjar syndrome |
|
Progressive familial intrahepatic cholestasis |
Hyperoxaluria |
|
Sclerosing cholangitis (primary and secondary) |
Gaucher's disease |
|
Caroli disease |
Familial hypercholesterolemia |
|
Congenital liver fibrosis |
Glycogenosis type IA |
|
Langerhans cell histiocytosis |
Protein C deficiency |
|
Organic acidemia |
|
|
Wolman's disease |
|
|
Metabolic disorders with cirrhosis |
Acute liver failure |
|
Alpha-1 antitrypsin deficiency |
Hepatitis |
|
Cystic fibrosis |
Neonatal hepatitis |
|
Wilson's disease |
Hepatitis B |
|
Tyrosinemia |
Hepatitis C |
|
Galactosemia |
Hepatitis non-ABC |
|
Neonatal hemochromatosis |
Autoimmune hepatitis |
|
Gestational alloimmune liver disease |
|
|
Glycogenosis type IV |
|
|
Niemann-Pick's disease |
|
|
Primary liver tumors |
Others |
|
Hepatoblastoma |
Budd-Chiari syndrome |
|
Hepatocellular carcinoma |
Cryptogenic liver cirrhosis |
|
Hemangioendothelioma |
|
|
Contraindications |
|
|
Rapidly progressing hepatocellular carcinoma with metastasis. Extra-hepatic malignancy Uncontrolled systemic infection Severe multisystem mitochondrial disease Niemann-Pick type C Severe Porto-pulmonary hypertension not responsive to medical therapy mPAP greater than 35 mmHg despite therapy |
|
mPAP: mean Pulmonary Artery Pressure
The PELD score ranks and prioritizes children by severity of ESLD in a single liver waiting list that includes adults according to their probability of death within 90 days of listing for LT [3,4]. Using objective and quantitative parameters, the PELD score (0-11 years) and MELD-Na + score (> 12 years) are used together to estimate the short-term waiting list mortality risk. In our center, we are trying to determine the priority to minimize the risk of mortality compared to the waiting list of the patients by making both scores [4,10,11].
Premedication
Anxiety and restlessness are incredibly high in these patients before the operation. For this reason, oral midazolam 0.25-0.5 mg/kg should be administered 30 min. before the operation, depending on the patient's condition and the risk of aspiration in these children. In addition, if the child has hypoxemia and cyanosis due to significant shunts and HPS, supplemental oxygen may be required preoperatively through a face mask or nasal cannula [11]. In our clinic, we apply midazolam anxiolysis to these patients in premedication according to the patient's condition, and we usually admit the patient to the operating room with his mother.
To ensure better positive postoperative results, the anesthesiologist should provide hemodynamics, fluid-electrolyte and acid-base balance, ventilation, perfusion, oxygenation, prevent factors that may trigger hypothermia, bleeding-coagulation, and keep pain under control during the operation [6,7,10-12].
Intraoperative Monitors, Anesthesia İnduction, and Management
Anesthesiologists should focus on rapidly fluctuating hemodynamics, physiology, metabolic, and coagulation status in the anesthesia management of these patients. Anesthetic induction in pediatric patients should be performed on a completely individualized case based on the etiology of the disease, related cardiopulmonary conditions, hemodynamic instability, fluid-electrolyte and acid-base disorders, amount of acid present, and history of esophageal varices. In these patients, anesthesia induction is provided intravenously (IV). The patient is pre-oxygenated at 80% for 3 minutes, but mask ventilation should be applied carefully in patients with ascites because of the risk of vomiting and aspiration. Propofol is generally used for induction of anesthesia. Although isoflurane is the recommended anesthetic agent because it can increase hepatic blood flow and maintain splanchnic blood flow, thereby better improving organ perfusion, other inhalation anesthetics such as sevoflurane or desflurane may also be preferred [8,9]. Rocuronium can be used as a muscle relaxant for rapid sequence induction, intubation (RSII), and subsequent infusion. RSII is a procedure that aims to reduce the incidence of pulmonary aspiration during airway management. RSII is achieved by minimizing the time between drug-induced loss of protective airway reflexes and a cuffed tracheal tube's successful insertion and inflation. Remifentanil is used as a continuous infusion during surgery as an analgesic [5-7,10,11].
In our clinic, we generally use propofol as an IV anesthetic agent, sevoflurane as an inhalation agent, rocuronium as a muscle relaxant, and remifentanil and rocuronium as a continuous infusion in most pediatric patients, including young infants. Intubation is performed with cuffed endotracheal tubes for safety. In our clinic, we generally prefer a pressure-guaranteed ventilation mode. We adjust the ventilation settings as the fraction of inspired oxygen (FiO 2 ), tidal volume 6-8 mL/kg, positive end-expiratory pressure (PEEP)3-5 cmH 2 O, and peak pressure < 20 mmHg according to the patient's needs. FiO 2 and pressures should be set to keep peripheral oxygen saturation (SpO 2 ) above 95% and partial arterial carbon dioxide (PaCO 2 ) between 35 and 45 mmHg. Insufficient PEEP can lead to alveolar collapse, and high PEEP may cause reduced vascular return, so caution should be taken [1,5,6,10-12].
Pediatric patients are highly susceptible to hypothermia in the intraoperative period due to peripheral vasodilation, frequent acid drainage bleeding, and existing malnutrition. Body temperature drops significantly with anesthetics and muscle relaxants, a cold environment, inadequate energy production from the liver, and bleeding. Hypothermia increases the bleeding tendency, causing hypoperfusion and metabolic acidosis with an accumulation of lactic acidosis resulting from anaerobic metabolism [12]. Hypothermia, acidosis, and coagulopathy cause the triad of death and should be avoided in any phase [10-12]. Also, the cold of the removed organ may exacerbate hypothermia and complicate the surgical process. Therefore, keeping patients as normothermic as possible during LT is vital to maintaining a normal physiological state, including cardiac, pulmonary, and coagulation cascade function. When the patient is admitted to the operating room, a heating blanket is placed under the patient. Heating methods (active and passive) should be applied throughout the operation, starting from the central catheterization and other preparation periods to ensure normothermia. Active normothermia (36°-37°) during the intraoperative period is the current standard of recommendation, and it is ideal for maintaining body temperature > 35-36 °C to prevent complications of hypothermia [10]. Our center tries to provide and maintain normothermia by mechanical ventilation with heat and moisture exchangers, heating blankets, steam heaters, convection air warming devices, warming the room, and all high-flow warming devices for IV fluid blood.
The anesthesia team comprises an anesthesia professor, an anesthesiologist, and a nurse. Unless there is a specific situation in the receiver, we begin with the donor graft in our center. What we do in our center within the monitoring scope is as follows. Standard ASA monitoring continuously measures physiological parameters such as BP, HR, SpO 2 , end-tidal carbon dioxide (EtCO 2 ), and body temperature [7,12,13].
Possible complications should be minimized when appropriate central, peripheral, and arterial cannulas are placed under ultrasonography (USG) guidance. It is critical to provide a thick and wide venous access line during LT, as there may be sudden massive bleeding that requires rapid blood product delivery. Therefore, two or more large-diameter peripheral IV cannulas should usually be placed in the upper extremities. Since the inferior vena cava (IVC) is clamped in the anhepatic phase, it should not be preferred too much as it may delay the delivery of IV blood, fluid, or drugs from the lower extremities to the central circulation. Suppose blood products are to be administered in infants and children younger than 12 months. Unlike adults, after administration of large blood products, pediatric patients have a higher risk of cardiac arrest due to electrolyte disturbances such as hypocalcemia and hyperkalemia; therefore, peripheral blood administration is preferred [5,6,10-13].
Catheterization is performed by choosing the right internal jugular vein under USG guidance. Monitoring proper arterial pressure (RAP) and administering vasopressors and inotropes is necessary. Central venous pressure (CVP) measurement gives us information about the trend of volume status [14,15]. Pulmonary artery catheter (PAC) use depends on the preference of the centers. However, considering the invasive method and the possibility of complications, we do not have PAC in our center. We prefer pulse contour cardiac output (PiCCO) monitoring. PiCCO, there is a need for continuous hemodynamic monitoring and evaluation of arterial blood gas (ABG). By placing a thermistor catheter in the femoral artery, static and dynamic parameters (SV, SVR, CO, cardiac index (CI), pulse pressure variation (PPV), stroke volume variation (SVV), global end-diastolic blood volume (GEDV), global end-diastolic volume index (GEDI), extravascular lung fluid (EVLW), and intrathoracic blood volume (ITBV). A central venous catheter is required for PiCCO use and must be inserted simultaneously. Sudden changes in intravascular volume should be measured by calibration [12-16].
Transesophageal echocardiography (TEE) is rarely used in pediatric LT compared to adult transplantation. TEE should be performed when necessary because it can help us diagnose and treat heart volume status and contractility, left ventricular outflow tract gradient, thrombus, pulmonary thromboembolism (PTE), pericardial effusion and tamponade, and unexplained hemodynamic instability [16,17]. In pediatric patients, TEE is contraindicated in cases such as severe thrombocytopenia, vascular ring, aortic arch anomaly with or without airway problems, tracheoesophageal fistula with unrepaired oropharyngeal pathology, esophageal obstruction or stenosis, and bleeding due to gastric and esophageal varices [16-19]. In intraoperative management, we apply TEE to the patient, paying attention to contraindicated when necessary. The novel, hands-free, transthoracic dual-plane echocardiography device allows for continuous, rapid assessment of volume status and cardiac function [17-19].
They suggested that the routine use of invasive arterial BP and CVP monitoring in minimum standard LT anesthesia should be applied according to specialist training in and using PAC, TEE, and/or transthoracic dual-plane echocardiography. It can provide invaluable guidance during a sudden cardiac collapse [17]. Our center's monitoring practice during LT is carried out in line with these recommendations.
Brain activity may slow down due to HE in ESLD [19]. Electroencephalography (EEG) monitoring should be performed when necessary to distinguish pathological changes in cerebral activity during anesthesia [11,12]. Bispectral Index (BIS) facilitates monitoring during the operation, measuring the anesthesia's depth and titration [19-21]. Muscle relaxant infusion can be used in LT; neuromuscular monitorization is appropriate due to residual neuromuscular block risks [12,21].
Due to bleeding-coagulation disorders with unlimited fluctuations throughout the LT, thromboelastography (TEG) and thromboelastometry (ROTEM) are invaluable tools that should be used to guide effective blood product and factor use Viscoelastic hemostatic analyses such as TEG and ROTEM are highly effective in directing perioperative blood management by reducing both the transfusion of allogeneic blood products and costs [21-23]. Traditional tests, such as Hb, prothrombin time (PT), fibrinogen, and the international normalized ratio (INR), and fibrinogen take a long time to give results, and the clinical picture can change quickly during this time. Our clinic uses TEG and ROTEM during the intraoperative period to make the right decisions about using blood products and factors.
Important intraoperative considerations in the surgical phases of LT are highlighted in Table 3. Therefore, it should include crucial perioperative patient management and approach, such as general rules, methods, and prevention of triggering factors that we should apply as anesthesiologists after appropriate preoperative evaluation and risk stratification. The things to be done in the perioperative approach in LT are summarized in Figure 1 [4-6,10-12,21].
Table 3: Anesthetic considerations, risk factors, and treatment during the pre-anhepatic, anhepatic, and reperfusion phases.
|
Pre-Anhepatic Phase |
||
|
Anesthetic considerations |
Risk Factors |
Treatment |
|
Massive hemorrhage |
Impaired synthetic function Dissection Varices Before abdominal surgery Adhesions Pre-existing coagulopathy Ascitic decompression |
Transfuse as necessary Vasopressors |
|
Hypotension |
Bleeding Large-volume ascites drained Acidosis Hypothermia |
5% Albumin for ascites May need vasopressor infusions |
|
Anhepatic phase |
||
|
Anesthetic considerations |
||
|
No production of clotting factors, fibrinogen deficiency |
||
|
Worsening coagulopathy progressive |
||
|
Hypocalcemia Absent citrate/lactate metabolism Worsening metabolic acidosis Reduced gluconeogenesis Surgical bleeding |
||
|
Reperfusion phase |
||
|
Anesthetic considerations |
||
|
Hypotension |
||
|
Reduce of SVR |
||
|
Sudden preload elevates at reperfusion |
||
|
Abrupt K+ increase at reperfusion |
||
|
Air embolism |
||
|
With possible arrhythmia or cardiac arrest |
||
SVR: Sistemic Vascular Resistance
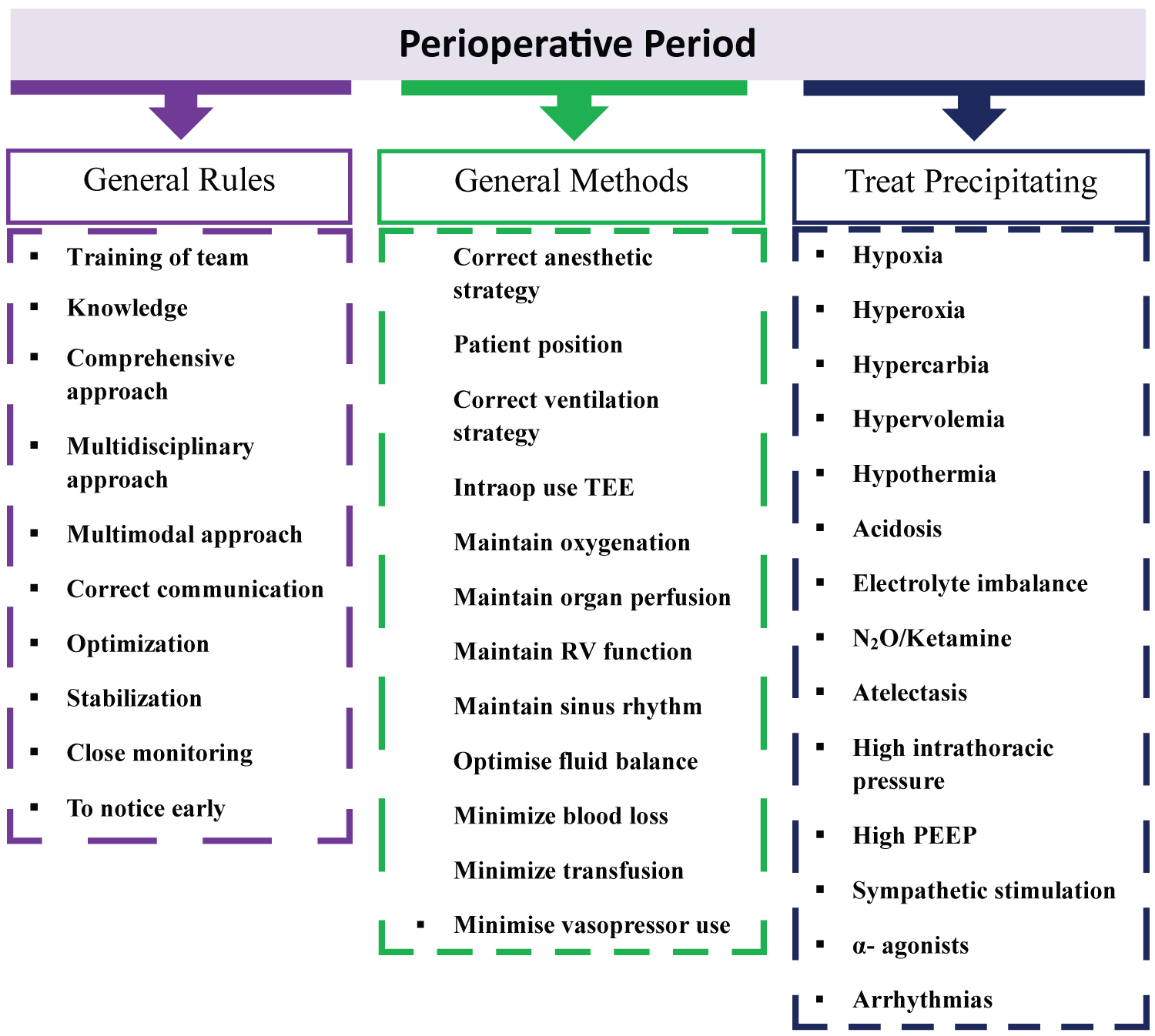
Figure 1:
The critical approach in the perioperative period for liver transplantation.
TEE: Transesophageal Echocardiography; RV: Right Ventricular; N2O: Nitrous Oxide; PEEP: Positive End-Expiratory Pressure
Since HE patients are more sensitive to sedative agents on LT, it is generally appropriate to avoid sedative premedication. RSII is performed as patients may have ascites and full stomachs. Fast-acting opioid (fentanyl 1-5 mg/kg) is used for induction. Propofol 0.3-1 mg/kg or etomidate 0.3-0.5 mg/kg is used. Intubation is performed with cricoid compression and a non-depolarizing muscle relaxant (1-1.5 mg/kg/hour of rocuronium bromide). In this patient, dose reduction should be made according to the current clinical situation and hemodynamics. Because serum albumin is low in these patients, protein binding decreases, and the free drug fraction increases [4,5,21]. After intubation, titration with remifentanil 0.02-0.2 µg/kg/min. In maintaining anesthesia, apply infusionand an inhaler (sevoflurane, with age-adjusted minimum alveolar concentration value) according to hemodynamics and BIS [10-12,21].
Pediatric LT presents many challenges for the anesthesiologist, requiring specialized knowledge, experience, and highly specialized intraoperative management. Many physiological changes may develop during LT that increase the risk of ESLD-related complications [4-7,10,11,14]. Each phase of LT produces dynamic changes, mainly due to factors such as hypovolemia, acute blood loss, abnormalities in CO and SVR due to ESLD, cross-clamping of the IVC and portal vein, and reperfusion of the liver [24]. At this stage, fluid and blood products and vasoactive drug managementare essential for anesthesia [24,25]. To monitor and treat hemodynamic changes closely due to these surgical maneuvers and fluid-blood loss during LT, circulatory data monitored with PiCCO can provide necessary guidance for volume management and vasoactive drug regulation to improve the perfusion of organs [12,13,25].
Human serum albumin is still the best colloidal solution in general fluid management during LT. Human albumin is given to expand the volume, retain fluid in the vein, and maintain oncotic pressure while increasing its concentration in children with protein levels and bulky acidity. The non-lactic acid compound sodium acetate is also a suitable crystalloid for LT [26]. Because of the potential for hyperchloric metabolic acidosis, normal saline is not recommended for patients with pediatric LT [27]. Our clinic uses human albumin 5% and a balanced crystalloid solution. Hyponatremia is a common finding in patients with ESLD and should not be corrected quickly because it causes central pons demyelination, which may result in cerebral edema and changes in consciousness. Therefore, it is more appropriate to use low Na + liquids [26-28]. The risk of hypoglycemia is higher during pediatric LT, so we routinely use 100 mL of 5% dextrose at baseline and adjust the rate to blood glucose levels with periodic monitoring of blood glucose levels. Our main goal should be to keep blood glucose between 4.7 and 6.9 mmol/L. There are also fundamental principles of blood product management during LT, which should be followed as much as possible. Red blood cell (RBC) suspensions should be administered to keep hematocrit (Hct) 24%-30% and hemoglobin (Hb) 8-10 g/dL within a specific range. It should be remembered that fresh frozen plasma (FFP), cryoprecipitate, and thrombocyte suspension should be given when the patient is given an amount of RBC greater than 20 mL/kg. Thrombocyte suspension is reserved for treating thrombocyte counts less than 25-50,000 cells/mL unless there is excessive surgical bleeding. In the presence of active bleeding/leakage, fibrinogen concentrate (25-50 mg/kg) with an MCF (maximum clot firmness) of < 10 mm according to FIBTEM, and fibrinogen > 150-200 mg/dL should be replaced with cryoprecipitate (1-2 U/10 kg) if there is no fibrinogen concentrate. No clear recommendation can be made regarding the indication for prothrombin complex concentrate (PCC) or FXIII, and using rFVIIa and desmopressin in the absence of hemophilia is not recommended. Intraoperative prophylactic administration of antifibrinolytics in pediatric LT is not advocated due to concerns of thrombosis [22,23]. TEG can provide helpful information and guide us in bleeding-coagulation situations. In the meantime, since triggering factors such as hypothermia and hypocalcemia can worsen bleeding, it should be tried to be avoided [21-23].
Perioperative unbalanced and inappropriate fluid management leads to organ hypoperfusion, and fluid overload causes peripheral and pulmonary edema. For this reason, patients should be kept normovolemic to ensure the organs' perfusion as much as possible. When choosing a fluid, the patient's fluid-electrolyte balance should be considered, and it should be kept in mind that iatrogenic hypernatremia, hyperchloremia, metabolic acidosis, and AKI may develop due to the use of isotonic saline and synthetic colloids [27-29]. In case of bleeding during LT during surgery, the operation area, the amount of blood in the aspirator, the number of sponges and tampons, and the cell saver should be considered. Blood products should be given according to Hb, PT, fibrinogen, and the INR, TEG, and ROTEM values. It should not be forgotten that using unnecessary and excessive allogeneic blood products increases morbidity and mortality [22].
Clamping the IVC at the end of the first phase can reduce SV, CO, and MAP, often not requiring correction. However, rapid correction may be required in case of a significant decrease in MAP and SV. Measurement of CVP can give us information about the course volume status, but this does not correlate with CO. Insufficient fluid administration may lead to hypotension and decrease the perfusion of the graft. Since hypervolemia may cause passive graft occlusion, CVP should generally be kept around 6-10 mmHg. Another way of measuring fluid response is the parameters SV, SVV, PVV, and GEDI. Volume loading may cause pulmonary edema, abnormal gas exchange, congestion, decreased perfusion, and graft edema. Monitoring with PiCCO as needed during all phases of LT provides many advantages in hemodynamic parameters and fluid management [15,16,25-27]. PICU stay, morbidity, and mortality can be reduced thanks to careful fluid management. When necessary, vasoactive drugs and adequate fluid therapy to protect the brain, heart, and kidney may provide better hemodynamic stabilization, CO, and perfusion [30-32]. In our clinic, fluid management is performed under the guidance of PiCCO.
Albumin is the essential protein that keeps fluids in the intravascular space and determines oncotic pressure. ESLD patients often have low albumin levels. Albumin in LT has been shown to reduce intraoperative fluid use and the frequency of pulmonary edema. At the same time, some studies have reported that albumin reduces the incidence of post-reperfusion syndrome (PRS), the use of vasopressor agents, and mortality in ESLD patients [2,28,30,32]. We prefer 5% albumin to retain fluid in the vessel, provide oncotic pressure and buffering effect, reduce protein loss and capillary permeability, and correct endothelial damage.
After vascular anastomoses, mannitol and furosemide are given to prevent congestion in the liver, edema in the abdominal organs and brain, and to provide diuresis. Mannitol should not be used routinely to provide diuresis. Mannitol is a hyperosmolar agent that increases serum osmolality, which causes water to move from swollen astrocytes into the serum. The use of mannitol is recommended only for acute increases in ICP and should not be used prophylactically [29]. Mannitol exerts an osmotic effect in the renal tubule and may help prevent ischemic injury. The current recommendation is to administer an IV bolus of 0.25-1.0 g/kg, which can be repeated once or twice as long as serum osmolality remains below 320 mOsm/L. The effect of mannitol is transient and should not be used if serum osmolality is > 320 mOsm/L in the presence of hypovolemia or renal failure. Excess volume may occur with mannitol and may require dialysis. Furosemide (0.5-1 mg/kg) acts as a Na + /K + pump inhibitor in the ascending cycle of Henle and may help to induce diuresis [29,30,33]. We administer mannitol and furosemide to prevent congestion and ischemic injury in the neo-hepatic phase after vascular anastomoses during LT.
Massive blood loss during LT is a common occurrence. In pediatric patients, cirrhotic coagulopathy, high PELD scores, portal hypertension, thrombocytopenia, and previous multiple abdominal surgeries are important risk factors for massive blood loss and should be considered [2,5,6,22,23]. Our clinic uses TEG and ROTEM during the intraoperative period to make the right decisions about using blood and blood products.
Cell saver is the technique of taking blood from the surgical field, washing and filtering it, then separating the RBC aside and giving it back to the patient when necessary. The benefit is that it reduces donors' use of allogeneic bank blood. However, contaminants such as intestinal and peritoneal microorganisms, denatured proteins or inflammatory proteins, fats, and malignant cells are also downsides, with concerns about repeat infusions, expensive equipment, and the need for installation by a trained person. Cell saver equipment is essential as the old technique of washing packed RBC can reduce the risk of hyperkalemia. Since the cell-saver blood does not contain coagulation factors, platelets, and plasma components, care should be taken to transfusion plasma products in case of rapid blood loss; otherwise, the coagulopathy will worsen, and the patient may develop hemorrhagic shock [22]. Nevertheless, we use cell savers during LT in our clinic.
LT surgery should be evaluated in 3 stages for better management. Criticaltargets are highlighted in Table 4 during the pre-anhepatic, anhepatic, and neo-hepatic phases [5,6,10,11,20,21].
Table 4: Critical targets during the pre-anhepatic, anhepatic, and reperfusion phases.
|
Pre-anhepatic phase |
|
|
Goal |
Target |
|
Maintenance Hemodynamic stability |
Keep MAP > 60 mmHg Keep CO > 5 L/min |
|
Maintenance coagulability |
Follow coagulation with TEG and INR |
|
Replacement blood or volume losses |
Maintain Hb > 8 gr/dL, platelets > 50000 mm3, fibrinogen > 150 mg/dL |
|
Avoidance of significant acidosis |
Maintain pH > 7.35 |
|
Avoid electrolyte derangements (K+ or Ca+2) |
Keep K+ low-normal (3.5-4.0 mmol/L) and ionized calcium > 1.2 mmol/L |
|
Normothermia and normoglycemia |
Expect ≈ 1° drop and keep blood glucose > 80 mg/dL |
|
Anhepatic phase |
|
|
Goal |
Target or Treatment |
|
Maintenance of intravascular volume and hemodynamic stability |
Hct 24%-30%, Hb 8-10 g/dL Keep CVP at low normal (7-10 mmHg) Use vasopressor infusions (norepinephrine or vasopressin) to augment BP and avoid over-transfusion/fluid administration To keep MAP > 60 mmHg and CO > 5 L/min |
|
Maintain normoglycemia |
May need dextrose bolus or infusion to keep blood glucose > 80 mg/dL |
|
Maintain normothermia |
Bair hugger, warming cap, warmed fluids, convection air warming devices, low-flow anesthesia |
|
Maintenance of acid-base balance |
Hyperventilation Sodium bicarbonate boluses to maintain near normal pH (1-2 mEq/kg) |
|
Maintain normal Ca++ and low-normal K+ levels |
Replace Ca+2 as necessary (Calcium chloride 10-20 mg/kg, central access or Calcium gluconate 30 mg/kg, central access or peripheral) To keep ionized calcium > 1.2 mmol/L Treat any hyperkalemia With hyperventilation (PaCO2 = 30 mmHg), albuterol (4 puffs), insulin (0.1 units/kg) with dextrose (0.25-0.5 g/kg), furosemide (0.5-1 mg/kg), sodium bicarbonate, and calcium chloride/gluconate, renal replacement therapy if available |
|
Reperfusion phase |
|
|
Goal |
Treatment |
|
Correct Ca++ |
Calcium chloride (central) 10-20 mg/kg IV |
|
Prevent or treat hyperkalemia Low-normal (K+ 3.5-4.0) |
Hyperventilation Calcium chloride boluses Sodium bicarbonate Insulin or dextrose Inhaled albuterol Furosemide Epinephrine |
|
Normothermia Expect ≈ a 1° drop |
Warm room Bair hugger Warm fluids |
|
Normotensive |
To keep MAP > 60 mmHg Norepinephrine and/or vasopressin To keep maintain CO > 5 L/min Dopamine and/or epinephrine When SVR is declining Phenylephrine 1-5 mg IV to keep MAP > 60 mmHg If necessary, methylene blue IV infusion (Keep in mind suspected vasoplegia) |
|
Normal HR |
If HR is < 60/min Epinephrine 20-100 µg boluses Defibrillation pads attached to defibrillator |
MAP: Mean Arterial Pressure; CO: Cardiac Output; TEG: Thromboelastography; INR: International Normalized Ratio; Hb: Hemoglobin; Hct: Hematocrit; CVP: Central Venous Pressure; SVR: Systemic Vascular Resistance; HR: Heart Rate; PaCO2: Partial Pressure of Carbon Dioxide
a) Pre-anhepatic Phase
The main aim of anesthesia management in this phase should be to provide hemodynamic stability, fluid-electrolyte balance, normovolemia, normothermia, normoglycemia, and prevent acidosis. Many hemodynamic and mechanical risk factors may trigger bleeding in the pre-anhepatic phase. Extensive dissection, portal hypertension, collateral circulation, adhesions due to previous multiple surgeries, cirrhotic coagulopathy, high PELD scores, thrombocytopenia, portal vein thrombosis (PVT), endothelial dysfunction (increase in NO and PGI 2 ), increases in conditions such as bacterial infection (endotoxemia, thrombocytopenia, increase in endogenous heparin-like agents) and kidney failure (anomalies in platelet structure and function, decreased adhesion and aggregation, abnormal platelet vessel wall interactions, deepening of anemia) are important risk factors for massive blood loss [19-23]. Profound hypotension with ongoing massive bleeding and acid drainage may lead to further deterioration of the patient with the addition of acidosis. To prevent hypotension and to provide intravascular volume, 5% or 20% albumin infusion should be started as an additional vasopressor with norepinephrine (NE) infusion without delay [27,28,30]. Anesthesiologists should perform the necessary and suitable fluid replacement to achieve normovolemia and avoid hypovolemia when clamping the IVC at the end of the pre-anhepatic phase [33]. Urine output (UO) and CVP are critical in monitoring intravascular volume. However, changes in intra-abdominal pressure and mechanical ventilation during surgery may be misleading in the accurate monitoring of CVP in the patient [12].
For this reason, it should be interpreted and decided by considering all hemodynamic findings while following the patient. It should be remembered that dilutional coagulopathy and thrombocytopenia may occur while trying to maintain the normal volume status [22,23,33]. Close and accurate communication between the anesthesiologist and the surgeon is crucial in evaluating all changes in the evaluation of the patient and the graft.
In our institution, we try to avoid unnecessary blood transfusions as much as possible by adhering to the transfusion policies. When looking at our data, 306 patients underwent living donors pediatric LT. A restrictive transfusion strategy should be applied. The effect of preoperative prophylactic transfusion has not been proven; the side effects of transfusion should be considered, the risk of hypervolemia should be considered, and as a result, increased PICU and hospital stay, mortality, morbidity, and cost. Early identification and treatment of bleeding and coagulopathy arecritical, and coagulation disorders should be corrected intraoperatively, not preop. Unnecessary transfusions should be avoided as much as possible, and the patient should be safe [20-23,33].
b) Anhepatic Phase
To facilitate hepatectomy and surgical vascular anastomoses, the IVC is clamped. The conventional method places vascular clamps on the intrahepatic and suprahepatic IVC. A popular alternative is the piggyback technique, where the IVC is partially or side-clamped to allow some blood flow during clamping, which may result in less hemodynamic instability. With complete or partial cross-clamping of the IVC in the anhepatic phase, hypotension is predominant, reducing the preload. Clamping the IVC can lead to severe hypotension with loss of up to 50% of the preload to the heart. Hypoglycemia may occur in pediatric patients due to metabolic acidosis, hypocalcemia, hyperkalemia, and incomplete functioning of gluconeogenesis. Supplementation with a continuous dextrose infusion and bolus, if needed, to keep blood glucose > 80 mg/dL. When metabolic acidosis develops, treatment with sodium bicarbonate (1 mEq/kg) to maintain near-normal pH. If hypocalcemia develops, supplementation with calcium chloride (10 mg/kg, central access) orcalcium gluconate (30 mg/kg, central access or peripheral) to keep ionized Ca ++ > 1.2 mmol/L. Also, aim for low normal K + levels (3-4 mEq/L) in preparation forreperfusion. Meanwhile, bleeding during anastomosis, a cold graft placed in the abdominal cavity, can deepen hypothermia. In this phase, the main anesthetic targets should be maintaining hemodynamic stability by providing adequate intravascular volume status, acid-base, fluid-electrolyte balance, normothermia, and normoglycemia. Close ABG follow-up is critical, especially for lactic acidosis [14,20,25,30,31]. In this stage, NE, vasopressin, epinephrine, dobutamine, dopamine, and phenylephrine are the most commonly used vasoactive drugs, alone or in combination. If vasoplegia continues despite multiple vasopressor applications, vasopressin is added as the first choice. In recent years, Methylene blue dye has been used successfully to treatpersistent vasoplegia. NE infusion should be titrated by fluid restriction to avoid edema while maintaining MAP. In treating resistant hypotension secondary to decreased SVR, in addition to NE, vasopressin or its synthetic analog terlipressin can be used as a vasoactive drug at a dose of 0.0001 U/kg/min [14,20,25,30,31]. In our clinic, we use NE in case of hypotension and vasopressin IV infusion when necessary. Hypotension is preferentially treated with vasopressors instead of IV fluids to avoid the risk of volume overload, right heart failure, and graft congestion during reperfusion. Our clinic's primary goals are fluid restriction, low/normal CVP (≤ 7 and 10 mmHg), and Hb (8-10 g/dL). To preserve perfusion to the liver graft, maintain MAP in the 50-70 mmHg range for infants, 60-80 mmHg for small children, or 70-90 mmHg for older children and adolescents. Therefore, administering appropriate doses of vasopressors may be more appropriate than excess fluid and transfusion because large amounts of fluid or blood product(s) may cause edema by causing congestion in the graft [14]. In addition, it is essential to provide hemodynamic optimization and end-organ perfusion.
c) Neo-hepatic Phase
Once the vascular anastomotic clamps are successfully released, the graft begins to receive blood again, and cold fluid, K + ions, cell debris, various pro-inflammatory cytokines, and small venous air emboli from the donor's liver can enter the recipient's circulatory system, causing severe hypotension, malignant ventricular arrhythmias, and even cardiac arrest [1,4-6,34,35]. When the IVC and portal vein are not clamped, venous return increases; however, inhibition of portal circulation, including mitochondrial energy loss, adenosine triphosphate depletion, Kupffer cell activation, calcium overload, oxidative stress, increased endogenous pro-inflammatory cytokines (IL-6 and TNF-a, K + , protons, and intrahepatic cold fluid/content), and it causes hemodynamic instability by rapidly passing varying amounts of embolic material into the patient's circulation. These mediators cause hemodynamic instability with abrupt reductions in MAP, HR, SVR, SV, EtCO 2 , and CO. These > 30% decreases in MAP and SVR hemodynamic changes are defined as PRS, and all necessary precautions should be taken to minimize the risk of PRS [34,35]. The surgical team should thoroughly wash the donor organ to avoid high blood flow and slowly unclamp the portal vein during the procedure [34-37]. In this phase, treating the existing hyperkalemia and metabolic acidosis quickly before reperfusion and anticipating and reducing the triggering risk factors such as hypothermia is significant. Insulin, hyperventilation (PaCO 2 ≈ 30 mmHg ) , IV calcium chloride, sodium bicarbonate, and furosemide may help treat hyperkalemia, and epinephrine can be repeatedly applied before reperfusion. At this stage, appropriate fluids, inotropes, and vasopressors are recommended to avoid overfilling. The anesthetics dose is reduced, and 100% oxygen is administered temporarily to prevent hypotension and hypoperfusion [36-38].
Meanwhile, steroids, N-acetylcysteine, vitamin C, and Mg ++ are recommended to reduce PRS formation. In addition, although it varies according to the practice of the clinics, bolus hydrocortisone (20 mg/kg) is administered when anhepatic phase is in our clinic for immunosuppression before liver reperfusion [35,36,38]. PRS increases perioperative morbidity and mortality, blood product transfusions, postoperative AKI, and ICU and hospital length of stay. PRS contributes to postoperative graft dysfunction, mainly affecting bile ducts that are highly susceptible to oxygen deprivation and reperfusion injury and can start a cascade that leads to multiple organ dysfunction [34-37]. Excess fluid resuscitation in this phase may lead to the development of hypervolemia in this period, resulting in deterioration in cardiopulmonary functions and congestion of the graft liver. This may result in prolonged weaning and pulmonary complications in the postoperative period [24,25,37].
Following reperfusion, hepatic artery and biliary tract anastomosis is completed. Following the anastomoses, hepatic/arterial and portal vein flows and resistances are checked with intraoperative Doppler USG. In addition, lactate levels are an essential marker in liver graft healing. It reaches a peak before reperfusion of the graft and then begins to decline gradually. The general trend in lactate monitoring is more important than absolute measured values and should, therefore, be considered more. However, a continuous progressive increase in lactate may be a sign of a severe condition [1,5,6,39,40]. Lactate elevation in ABG and Doppler USG should be evaluated and interpreted together, and patient management should be performed according to the possible situation.
Hypothermia, acidosis, hypocalcemia, hyperkalemia, and other electrolyte disturbances should be prevented at all stages during surgery. Since there will be immediate and fluctuating hemodynamic changes, close monitoring and measurement of ABG should be performed.
It should include crucial perioperative patient management and approach, such as general rules, methods, and prevention of triggering factors that we should apply as anesthesiologists after appropriate preoperative evaluation and risk stratification (Figure 1) [4-6,10-12,21].
d) Neo-hepatic Phase
In this phase, after reperfusion, coagulopathy, continuous blood losses from the vascular anastomosis, and the surface of the partial donor graft can be observed. Careful fluid management should be performed to prevent graft occlusion due to fluid overload. Unlike adult patients, in the post-reperfusion stage, jejunum = roux hepatico-jejunostomy or choledocholedocostomy is performed from the bile duct in pediatric patients. When the graft organ starts to work, the hemodynamics become more stable, and the acid-base status and electrolyte disturbances improve. One of the most critical conditions after reperfusion in pediatric LT is the possibility of vascular thrombosis due to a decrease in protein C, protein S, plasminogen, and antithrombin III levels and an increase in factor VIII [38-40]. According to the Cochrane review, in these patients, the incidence of hepatic artery thrombosis (HAT) after pediatric LT greatly varies from center to center, ranging from 5% to 18%, while portal venous thrombosis (PVT) occurs in 5%-10% of recipients. A mortality rate of HAT 25% has been reported in pediatric LT [40]. Causes that increase the risk of HAT increase with risk factors such as more significant artery size inconsistency, lower patient weight, and perfusion due to higher blood viscosity and higher Htc, reoperation, and longer surgery times. Acetylsalicylic acid, heparin, and PGE-1 should be initiated in the treatment when HAT develops. In our clinic, we start anticoagulant therapy in addition to surgery in cases of HAT detected by Doppler USG without wasting time [41-43]. Abdominal closure should be cautiously performed, especially in small infant patients or cases of larger graft organ size with a higher risk of graft displacement and compression. In cases with excessive abdominal compression, gradual abdominal closure can be performed [43,44].
Perioperative Extubation
At the end of LT, if the hemodynamics of the patient are stable, the bleeding is not excessive, the operation time is short, and the extubation criteria are met, extubation can be performed in the operating room, or the patient can be extubated in the PICU in a controlled manner depending on the severity of the problems experienced in the intraoperative period. Extubation in the operating room relies not only on the transplant center, the experience of the surgeon, and the excellent and accurate follow-up of the anesthetists but also on the patient's indication for LT, comorbidities, and graft-related factors. If all relevant factors are carefully considered and conditions are suitable for safe extubation, the patient can be successfully performed in the operating room [45,46]. Considering all these factors in our clinic, we usually extubate patients in the operating room and the PICU if possible risk factors exist.
Early Postoperative Phase
The most important goals in the early postoperative phase are to provide hemodynamic stability and maintain graft function. Due to the risk of thrombosis, maintenance of > 25% Htc should be avoided during this phase. Hypovolemia usually causes a decrease in UO; however, poor graft function and risk factors such as poor preoperative kidney function, massive blood transfusion, and sepsis can cause oliguria and lead to early kidney dysfunction. Therefore, appropriate intravascular filling and CO are required to maintain renal perfusion and diuresis. However, excessive fluid administration, pleural effusion, and large graft size may affect respiratory mechanics in the patient, leading to oxygenation impairment [47-49]. In the postoperative period, the vascular status of the liver must be checked with Doppler USG [40].
Postoperative Management in PICU
Acute management after surgery includes extubation in the operating room, hemodynamic findings, fluid-electrolyte and acid-base balance, diuresis and pain management, and communication between anesthesiologist and surgeon for planning other treatments. Intubated patients in the PICU are usually extubated within 48 hours, if appropriate, according to the patient's clinical and hemodynamic status [45,46]. After LT, patients should be followed closely in the PICU for respiratory, cardiovascular, neurological, hematological, and significant complications. All patients should be followed up with standard monitoring and invasive BP and CVP measurements. The sedation score should be evaluated before starting any sedation or pain medication treatment, whether extubated or intubated in the PICU [48,49]. Consciousness level is an accurate parameter in evaluating the function of the newly transplanted liver in the postoperative period and should be done at intervals, and sedation should be started afterward. The patient should be sedated to prevent excessive sedation when the Richmond Agitation-Sedation Scale (RAASS) score is -2 or -3. If the patient is on a ventilator, the infusion can usually be started with remifentanil, propofol (> 6-years-old), and the muscle relaxant rocuronium 0.2-0.5 mg/kg/hour [1,4,5,48]. In our clinic, we generally prefer remifentanil infusion and do not give muscle relaxant infusion as much as possible. In the early postoperative period, benzodiazepines that undergo hepatic metabolism should be avoided. Conditions such as atelectasis, pleural effusion, pulmonary edema, elevated CO 2 production, or mechanical causes or infection in the airway should be considered in patients requiring longer mechanical ventilator support. However, decreased ventilation capacity because of respiratory depression caused by liver dysfunction, metabolic disorders, and malnutrition in oversedated patients may also contribute to the need for elongated ventilation support. Patients risk ulceration in the stomach and duodenum due to increased stress, steroid, and immunosuppressive treatment in the post-transplant period. Therefore, gastric acid secretion inhibitors should be given to all patients. Anesthesiologists should be focused on in the PICU, postoperative hemodynamic optimization and stabilization, management of fluid-electrolytes balance (due to prolonged operation and massive fluid shifts), management of graft rejection, infection prophylaxis, administration of calcineurin inhibitors, follow-up and correction of hypothermia, coagulopathy, early weaning and renal function in the patient who has not yet been weaned from the mechanical ventilatoris protection. It has been reported that combining a fluid-limiting approach and early extubation can improve hepatic function rapidly [39,45-47].
Elevated body temperature due to increased metabolism, hyperglycemia due to increased gluconeogenesis, normalization of coagulopathy, bile formation, and improvement of acidosis and lactate levels are early signs of improved graft function. Anticoagulation therapy is indicated to prevent thrombosis of hepatic vessels unless contraindicated in all LT patients in the postoperative period. Initiation criteria should be started immediately if INR < 2, platelets > 50.000. If no blood is coming from the drains, talk to the surgical team; it should be started sooner if the patient is at high risk after consulting the surgeons. Meanwhile, the drains should be emptied every 2 hours, the amounts should be recorded, and any change in the color and/or amount of the drain outlet should be immediately evaluated by the surgical team. Patients after LT should be evaluated by the comprehensive ERAS protocol and followed accordingly [47-50].
Immunosuppression
After pediatric LT, immunosuppressive agents should be significantly accurate and appropriate to prevent allograft rejection and minimize the risk of infection. Managing immunosuppression and using these agents in pediatric patients is often more complex and challenging than using them in adult LT patients. Physiological differences, pharmacokinetics, and pharmacodynamics in pediatric patients' properties alter the features of immunosuppressive agents. Pediatric patients have a longer duration of exposure to immunosuppression because of many causes, including the risk of infection (bacterial, viral, and fungal), growth, carcinogenesis, and the possibility of non-adherence to treatment. The dose of immunosuppressive agents after LT is adjusted according to blood levels, functional status of the transplanted liver, and kidney function [49,51]. In the intraoperative period, hydrocortisone or methylprednisolone (20 mg/kg) is given immediately before graft reperfusion, and 5 mg/kg divided into four doses is continued the next day. After that, the dose is reduced to 5 mg daily and changed to 5 mg prednisone for at least one year or half dose in young children and continued [49,50]. In addition to steroids, the first-choice calcineurin inhibitor is the immunosuppressive drug tacrolimus (FK 506), and tacrolimus inhibits the action of interleukin-2, which activates T lymphocytes, a central component in transplant rejection. It is usually given in divided doses of 0.15 mg/kg, titrated to tacrolimus levels between 8 and 12 µg/L during the first few weeks. Tacrolimus is a potentially nephrotoxic drug with many side effects such as nausea-vomiting, hypervolemia, hypertension, hypernatremia, hyperkalemia, hyperglycemia, hypomagnesemia, oliguria, central nervous system disorders, seizures, anemia, in toxic levels. In patients who develop delayed AKI caused by calcineurin inhibitors, doses of tacrolimus can be decreased by combining it with other drugs with the same effects and fewer nephrotoxic effects, such as mycophenolate and sirolimus. In addition, antibiotic prophylaxis after LT should be routinely applied to all patients [49-52].
Postoperative Complications
LT is one of the most hemorrhagic surgical interventions. Because of the significant feature of the surgery and the disease's effect on many organs, postoperative complication rates are incredibly high and critical in organ dysfunction, immunosuppression, and infections [52]. These patients need close follow-up support in the postoperative period. Doppler USG imaging should be performed twice daily to see anastomoses, vascular structures, and perfusion in the first 48 hours after surgery. Daily Doppler USG imaging should be performed in 5 days. If arterial/venous flow signals are not observed or decreased by Doppler USG, Computed tomography (CT) may be required by contacting the surgeons [53]. Post-LT complication rates are higher in children and the early detection and treatment of complications that may develop after LT are essential in providing low mortality and morbidity rates (Table 5) [48-55]. Early and rapid complications diagnosis is vital for patient survival but is challenging given the lack of specificity in clinical presentation. Vascular and biliary complications are the most common, but parenchymal and extra-parenchymal abdominal complications occur more rarely [56,57]. The surgical complications of LT may be classified as venous, arterial, or biliary. The most common complications are due to bile, whereas vascular complications are critical for graft viability. Due to the broad clinical symptoms and nonspecific findings, imaging results are vital to diagnosing these complications. Magnetic resonance imaging (MRI) diagnoses biliary complications, bile leaks, and neurological complications [52-55]. Biliary leakage was our center's most common indication for early relaparotomy.
Table 5: Postoperative complications after liver transplantation.
|
Vascular complications |
Hepatic artery complications Hepatic arterial thrombosis (HAT) Hepatic arterial stenosis (HAS) Hepatic artery pseudoaneurysm Arterioportal fistula and celiac artery stenosis |
|
Portal vein complications Portal vein thrombosis (PVT) Portal vein stenosis |
|
|
Hepatic veins and inferior vena cava complications Thrombosis Stenosis |
|
|
Splenic artery steal syndrome (SASS) Liver ischemia/infarction |
|
|
Biliary complications |
Stricture, fistula, sphincter of Oddi dysfunction Stone/cast/sludge, biliary cast syndrome Bile leak Ductal ischemia Recurrent biliary disease Mucocele of cystic duct remnant |
|
Fluid collections |
Seroma Hematoma Abscess |
|
Abdominal complications |
Bleeding Bowel obstruction Internal and external hernias Perforation Pneumatosis intestinalis Mesenteric ischemia Stercoral and infective colitis Malposition of catheters |
|
Pulmonary complications |
Pleural effusion, atelectasis, pneumonia (bacterial, viral, and fungal), pulmonary edema, acute respiratory distress syndrome (ARDS), alveolar hemorrhage, malposition of tubes and catheters |
|
Neurological Complications |
Hepatic encephalopathy Cerebral edema Central nervous system infections Central pontine and extrapontine myelinolysis Acquired hepatocerebral degeneration Seizures Posterior reversible encephalopathy syndrome (PRES) |
|
Neoplasms |
|
|
Rejection |
The postoperative period in patients undergoing LT fluctuates, and the results are multifactorial, including patient condition, graft organ, and technical problems in operation. Timely diagnosis and treatment of possible complications in the postoperative period are critical in minimizing morbidity and mortality and improving outcomes. Otherwise, untreatable complications after LT can significantly increase adverse outcomes and hospital costs [47,48,52-55].
Postoperative Pain Management
In the post-LT period, comprehensive and multimodal pain management consisting of parental education and standard pharmacological and non-pharmacological techniques is vital for the patient to have a comfortable, pain-free period. For this purpose, multimodal analgesia, patient-controlled analgesia, and anxiolytics are appropriate and are the basis of pain management. The most preferred opioid drugs in treatment are hydromorphone and tramadol. In line with the ERAS protocol, analgesic options such as paracetamol, dexmedetomidine hydrochloride, and ketamine can be used to avoid excessive opioid treatment and reduce the side effects of these drugs [47,48,58,59]. The main goal in pain management is to be managed and monitored by the hospital's Algology team. In our center, pain management is monitored by the algology team with multimodal analgesia methods.
Nutrition
Nutrition is highly critical in increasing recovery in the post-LT period. In these patients, enteral feeding is started on the second or third postoperative day for duct-to-duct anastomosis and on the fourth or fifth day for Roux-en-Y hepaticojejunostomy. When all factors are considered, achieving complete nutrition in practical children will be on the seventh or eighth day [2,4-6,20,21,47]. One day, parenteral nutrition should be started in patients with malnutrition and special metabolic conditions. Since there will be a rapid growth stage after LT, it is essential to supplement the diet with appropriate vitamins and minerals. Extra calories and protein support are essential as they aid in wound healing, fighting infection, and regaining weight. Adequate, correct, and healthy eating habits can significantly affect the patient's long-term health. Recovery after LT is completed 6 to 12 weeks after surgery [47,60,61]. The patient's nutritional status in our center is evaluated by the intensivist, surgeon, nutrition committee, family, and clinic.
LT is a highly complex process involving multiple changes and complications. It is always a dynamic process, from the decision to LT to the end. Many transplant centers have an LT protocol applied. The standard LT anesthesia protocol we apply in our clinic is summarized in Table 6. The most critical steps in LT are a multisystemic approach, the ability to manage the patient well, fast, effective, and accurate communication, and implementing protective and preventive strategies (Figure 2).
Table 6: Liver transplantation protocol in our clinic.
|
Monitoring |
Induction |
|
ECG |
is evaluated according to the patient |
|
SpO2 |
Propofol 0.3-1 mg/kg or etomidate 0.3-0.5 mg/kg IV |
|
EtCO2 |
Fentanyl 1-5 µg/kg bolus |
|
Peripheral venous lines |
Remifentanil 0.02-0.2 µg/kg/min infusion |
|
Arterial cannula (Radial arterial ) |
Sevoflurane, desflurane |
|
CVP (Vena jugularis interna ) |
Rocuronium bromide 0.6 mg/kg bolus |
|
PICCO (Femoral arterial ) |
Antibiotic therapy |
|
Heat probe |
PPI |
|
Nasogastric tube |
|
|
Urinary catheter |
|
|
TOF |
|
|
BIS |
|
|
Anesthesia maintenance |
|
|
Sevoflurane/Desflurane |
|
|
Remifentanil: 0.01-0.2 mg/kg/min. IV infusion |
|
|
Rocuronium bromide: 0.3 mg/kg/h IV infusion |
|
|
Norepinephrine: 0.01-0.1 mg/kg/min. IV infusion |
|
|
Fluid: Isolyte-S + 5% Albumin + 5% Dextrose |
|
|
Dissection Phase |
|
|
Heparin: 0.3 mg/kg |
|
|
Anastomosis follows Hepatic vein-portal vein (the cross-clamp is removed at the end) and hepatic arterial/Choledoc duct. |
|
|
Mannitol: 0.5 gr/kg |
|
|
Anhepatic phase |
|
|
Steroid: 20 mg/kg (before the recipient's anastomoses start) + PPI |
|
|
N-Acetylcysteine (NAC): 100 mg/kg (minimum infusion in 30 min.) |
|
|
Reperfusion Phase |
|
|
Coagulation parameters and complete blood count (Hb, Hct) are seen 1 hour after cross-clamp removal. |
|
|
When the arterial anastomosis is finished |
|
|
Vitamin C: 500 mg, child 1/4 or 1/5 dose |
|
|
According to urine output 15 minutes after furosemide-mannitol, if necessary |
|
|
Rocuronium bromide infusion is stopped |
|
|
Postoperative Analgesia |
|
|
Child: Morphine PCA (0.1 mg/h infusion, bolus not given) |
|
|
An increasing number of ABG evaluations are performed as needed, at baseline and in each phase |
|
|
PiCCO measurement is made at basal and in each phase |
|
LT: Liver Transplantation; ECG: Electrocardiography; SpO2: Peripheral Oxygen Saturation; EtCO2: End-Tidal Carbon Dioxide; CVP: Central Venous Pressure; PiCCO: Pulse Contour Cardiac Output; TOF: Train of Four; BIS: Bispectral Index; PPI: Proton Pump Inhibitor; PCA: Patient-Controlled Analgesia; ABG: Arterial Blood Gas
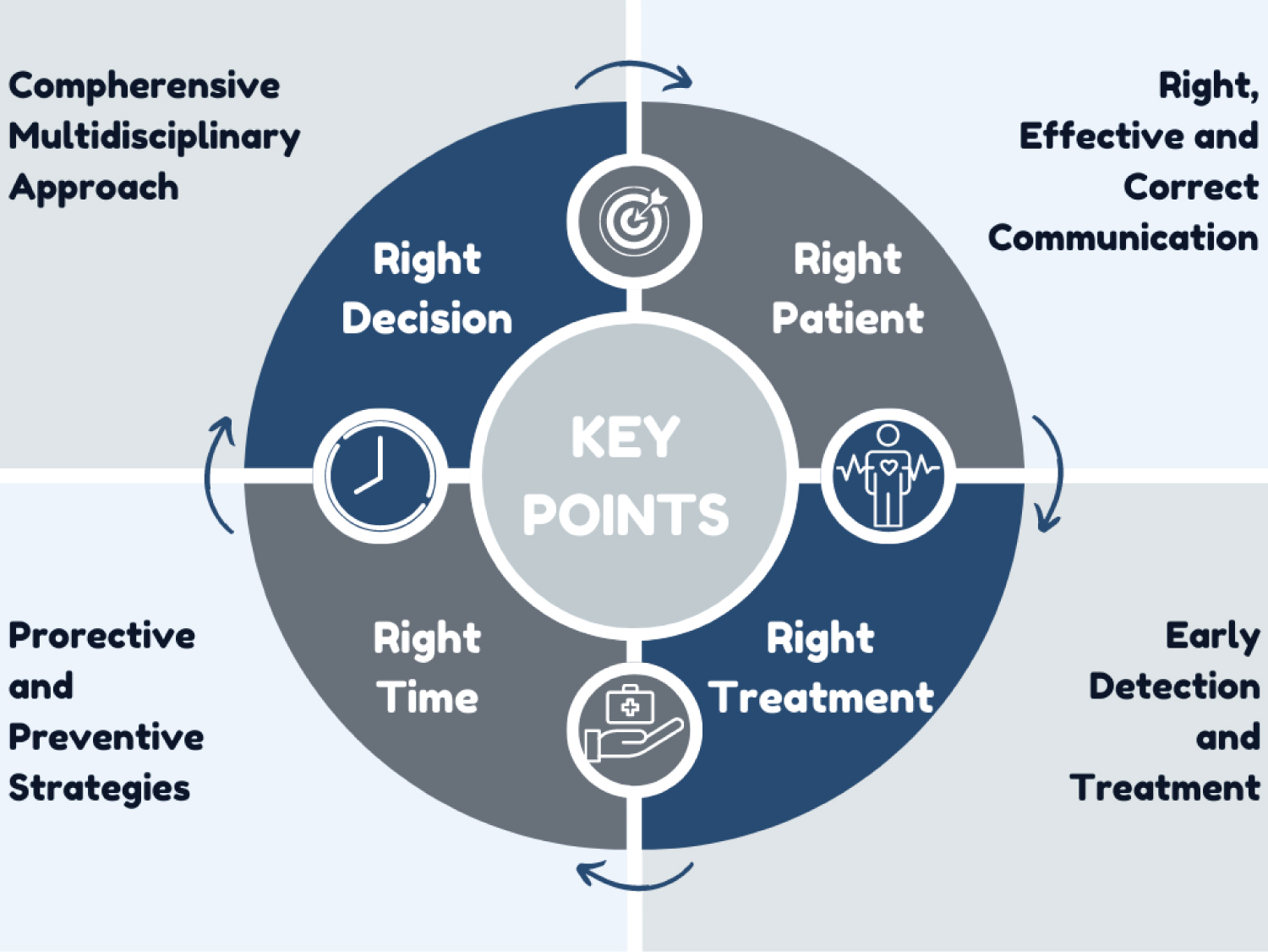
Figure 2: Critical points in the perioperative period for liver transplantation.
Conclusion
Proper anesthesia management in pediatric LT depends on a comprehensive understanding, knowledge, attitude, and practice of the effects of ESLD-related physiological and metabolic changes on organs in the perioperative period. In these patients, a multidisciplinary approach, detailed evaluation, and optimization are essential in the perioperative period. Anesthesia management should focus on rapidly changing physiological, hemodynamic, metabolic, coagulation, and bleeding changes. At the same time, they should receive adequate training on transplantation anesthesia, follow protocols and guidelines closely, and act accordingly. The most basic and vital aspect of patient management is accurate and close communication between the anesthesiologists and the surgical team.
Funding
No funding.
Acknowledgments
None.
Declaration of Interest
None.
Authors Contribution
AU acquired the table and figures and produced the first draft of this review. NÇ reviewed and polished the manuscript.
References
- Dhawan A, Shanmugam N. Paediatric liver transplantation: perioperative management. In: Pediatric liver intensive care. Singapore: Springer Singapore, 2019:95-105.
- Cuenca AG, Kim HB, Vakili K. Pediatric liver transplantation. Semin Pediatr Surg 2017;26:217-223.
- Nelson J, Cladis FP. Pediatric liver transplantation. Lalwani K, Cohen IT, Choi EY, Raman VT, Oxford University Press, 2018.
- Squires RH, Ng V, Romero R, et al. Evaluation of the pediatric patient for liver transplantation: 2014 practice guideline by the american association for the study of liver diseases, american society of transplantation and the north american society for pediatric gastroenterology, hepatolo. Hepatology 2014;60:362-398.
- Kim EJ, Koo B-N. Anesthetic management in pediatric liver transplantation. Korean J Transplant 2018;32:31-37.
- Wasson NR, Deer JD, Suresh S. Anesthetic management of pediatric liver and kidney transplantation. Anesthesiol Clin 2017;35:421-438.
- Khemakanok K, Khositseth A, Treepongkaruna S, et al. Cardiac abnormalities in cirrhotic children: pre- and post-liver transplantation. Hepatol Int 2016;10:518-524.
- Drobish JK, Reina E, Nieva D, et al. Outcomes following formation of a dedicated pediatric liver transplant anesthesia team. Pediatr Anesth 2022;32:732-739.
- Fernandez TMA, Schofield N, Krenn CG, et al. What is the optimal anesthetic monitoring regarding immediate and short-term outcomes after liver transplantation?-A systematic review of the literature and expert panel recommendations. Clin Transplant 2022;36.
- Sharma V, Kleb C, Sheth C, et al. Cardiac considerations in liver transplantation. Cleve Clin J Med 2022;89:46-55.
- Whitney GM, Chatterjee D. Error traps and culture of safety in pediatric anesthesiology. Pediatr Anesth 2021;31:258-259.
- Hendrickse A, Crouch C, Sakai T, et al. Service requirements of liver transplant anesthesia teams: society for the advancement of transplant anesthesia recommendations. Liver Transplant 2020;26:582-590.
- Magnée C de, Veyckemans F, Pirotte T, et al. Liver and systemic hemodynamics in children with cirrhosis: Impact on the surgical management in pediatric living donor liver transplantation. Liver Transplant 2017;23:1440-1450.
- Ballard HA, Jones E, Malavazzi Clemente MM, Damian D, Kovatsis PG. Educational review: Error traps in anesthesia for pediatric liver transplantation. Pediatr Anesth 2022;32:1285-1291.
- Wu C-Y, Cheng Y-J, Liu Y-J, Wu T-T, Chien C-T, Chan K-C. Predicting stroke volume and arterial pressure fluid responsiveness in liver cirrhosis patients using dynamic preload variables. Eur J Anaesthesiol 2016;33:645-652.
- Rocca G Della, Chiarandini P. Hemodynamic monitoring during liver transplantation. Int Anesthesiol Clin 2017;55:121-134.
- Porter TR, Shillcutt SK, Adams MS, et al. Guidelines for the use of echocardiography as a monitor for therapeutic intervention in adults: A report from the american society of echocardiography. J Am Soc Echocardiogr 2015;28:40-56.
- Ashley M, Justin M. Predicting and measuring fluid responsiveness with echocardiography. Echo Res Pract 2016;3:G1-12.
- Brezeanu LN, Brezeanu RC, Diculescu M, Droc G. Anaesthesia for liver transplantation: An update. J Crit Care Med 2020;6:91-100.
- Tran LT, Carullo PC, Banh DPT, Vitu C, Davis PJ. Pediatric liver transplantation: Then and now. J Cardiothorac Vasc Anesth 2020;34:2028-2035.
- Paterson NAB, Lee-Archer P, Shirley A, Lee J. Pediatric liver transplantation in Australia and New Zealand: The case for a collaborative anesthetic database. Pediatr Anesth 2021;31:309-315.
- Kozek-Langenecker SA, Ahmed AB, Afshari A, et al. Management of severe perioperative bleeding. Eur J Anaesthesiol 2017;34:332-395.
- Bezinover D, Dirkmann D, Findlay J, et al. Perioperative coagulation management in liver transplant recipients. Transplant 2018;102:578-592.
- Chen P, Chan K, Liao M, Wu C. Accuracy of dynamic preload variables for predicting fluid responsiveness in patients with pediatric liver cirrhosis: A prospective study. Pediatr Anesth 2020;30:455-461.
- Sanford EL, Zurakowski D, Litvinova A, Zalieckas JM, Cravero JP. The association between high-volume intraoperative fluid administration and outcomes among pediatric patients undergoing large bowel resection. Pediatr Anesth 2019;29:315-321.
- Efune PN, Hoyt MJ, Saynhalath R, et al. Intraoperative fluid administration volumes during pediatric liver transplantation and postoperative outcomes: A multicenter analysis. Pediatr Anesth 2023;33:754-764.
- Carrier FM, Chassé M, Wang HT, et al. Restrictive fluid management strategies and outcomes in liver transplantation: a systematic review. Can J Anaesth 2020;67:109-127.
- Orbegozo Cortés D, Gamarano Barros T, Njimi H, Vincent J-L. Crystalloids versus colloids. Anesth Analg 2015;120:389-402.
- McLean DJ, Shaw AD. Intravenous fluids: effects on renal outcomes. Br J Anaesth 2018;120:397-402.
- Boer C, Bossers SM, Koning NJ. Choice of fluid type: Physiological concepts and perioperative indications. Br J Anaesth 2018;120:384-396.
- Kumba C, Willems A, Querciagrossa S, et al. A systematic review and meta-analysis of intraoperative goal directed fluid and haemodynamic therapy in children and postoperative outcome. J Emerg Med Critical Care. 2019;5(1):1-9.
- Ferrario M, Pala S, Aletti F, et al. Fluid responsiveness in liver surgery: comparisons of different indices and approaches. J Comput Surg 2014;1:6.
- Bezinover D, Mukhtar A, Wagener G, et al. Hemodynamic instability during liver transplantation in patients with end-stage liver disease: A consensus document from ILTS, LICAGE, and SATA. Transplant 2021;105:2184-2200.
- Bulkley GB. Reactive oxygen metabolites and reperfusion injury: aberrant triggering of reticuloendothelial function. The Lancet 1994;344:934-936.
- Bukowicka B, Abi Akar R, Olszewska A, Smoter P, Krawczyk M. The occurrence of postreperfusion syndrome in orthotopic liver transplantation and its significance in terms of complications and short-term survival. Ann Transplant 2011;16:26-30.
- Rampes S, Ma D. Hepatic ischemia-reperfusion injury in liver transplant setting: mechanisms and protective strategies. J Biomed Res 2019;33:221.
- Fukazawa K, Yamada Y, Gologorsky E, Arheart KL, Pretto EA. Hemodynamic recovery following postreperfusion syndrome in liver transplantation. J Cardiothorac Vasc Anesth 2014;28:994-1002.
- Jeong S-M. Postreperfusion syndrome during liver transplantation. Korean J Anesthesiol 2015;68:527.
- Craig EV, Heller MT. Complications of liver transplant. Abdom Radiol (NY) 2021;46:43-67.
- Hugenholtz GCG, Northup PG, Porte RJ, Lisman T. Is there a rationale for treatment of chronic liver disease with antithrombotic therapy? Blood Rev 2015;29:127-136.
- Nacoti M. Coagulopathy and transfusion therapy in pediatric liver transplantation. World J Gastroenterol 2016;22:2005.
- Neto JS, Fonseca EA, Vincenzi R, et al. Technical choices in pediatric living donor liver transplantation: the path to reduce vascular complications and improve survival. Liver Transplant 2020;26:1644-1651.
- Heaton ND. Hepatic artery thrombosis: Conservative management or retransplantation? Liver Transplant 2013;19:S14-S16.
- Feltracco P. Perioperative thrombotic complications in liver transplantation. World J Gastroenterol 2015;21:8004.
- Saeyup P, Paarporn P, Prasarnphan D, Wongpiyaboworn W. Factors associated with immediate and early extubation in pediatric living-donor liver transplant recipients. Pediatr Anesth 2023;33:59-68.
- Gurnaney HG, Cook-Sather SD, Shaked A, et al. Extubation in the operating room after pediatric liver transplant: A retrospective cohort study. Pediatr Anesth 2018;28:174-178.
- Brustia R, Monsel A, Conti F, et al. Enhanced recovery in liver transplantation: A feasibility study. World J Surg 2019;43:230-241.
- Brustia R, Monsel A, Skurzak S, et al. Guidelines for perioperative care for liver transplantation: Enhanced recovery after surgery (ERAS) recommendations. Transplant 2022;106:552-561.
- Covarrubias K, Luo X, Massie A, et al. Determinants of length of stay after pediatric liver transplantation. Pediatr Transplant 2020;24.
- Tannuri U, Tannuri ACA. Postoperative care in pediatric liver transplantation. Clinics 2014;69:42-46.
- Miloh T, Barton A, Wheeler J, et al. Immunosuppression in pediatric liver transplant recipients: Unique aspects. Liver Transplant 2017;23:244-256.
- Karjoo M, Kiani MA, Arash S, Saeidi M. Short and long term complications after pediatric liver transplantation: A review and literature. Int J Pediatr 2017;5:6337-6346.
- Sureka B, Bansal K, Rajesh S, Mukund A, Pamecha V, Arora A. Imaging panorama in postoperative complications after liver transplantation. Gastroenterol Rep (Oxf) 2016;4:96-106.
- Horvat N, Marcelino ASZ, Horvat JV, et al. Pediatr liver transplant: Techniques and complications. Radio Graphics 2017;37:1612-1631.
- Laurence JM, Sapisochin G, DeAngelis M, et al. Biliary complications in pediatric liver transplantation: Incidence and management over a decade. Liver Transplant 2015;21:1082-1090.
- Bezinover D, Deacutis MF, Dalal PG, et al. Perioperative thrombotic complications associated with pediatric liver transplantation: A UNOS database evaluation. HPB 2019;21:370-378.
- Stine JG, Pelletier SJ, Schmitt TM, Porte RJ, Northup PG. Pre-transplant portal vein thrombosis is an independent risk factor for graft loss due to hepatic artery thrombosis in liver transplant recipients. HPB 2016;18:279-286.
- Feltracco P, Carollo C, Barbieri S, et al. Pain control after liver transplantation surgery. Transplant Proc 2014;46:2300-2307.
- Vittinghoff M, Lönnqvist P, Mossetti V, et al. Postoperative pain management in children: Guidance from the pain committee of the European Society for Paediatric Anaesthesiology. Pediatr Anesth 2018;28:493-506.
- Pawlowska J. The importance of nutrition for pediatric liver transplant patients. Clin Exp Hepatol 2016;3:105-108.
- Mortensen M, Lundberg C, Horslen S. Nutritional management for infants and children pre and post-liver transplant. OBM Transplant 2018;3:1-1.
Table of Contents
- Abstract
- Keywords
- Introduction
- Preoperative Assessment
- Premedication
- Intraoperative Monitors, Anesthesia İnduction, and Management
- Perioperative Extubation
- Early Postoperative Phase
- Postoperative Management in PICU
- Immunosuppression
- Postoperative Complications
- Postoperative Pain Management
- Nutrition
- Conclusion
- Funding
- Acknowledgments
- Declaration of Interest
- Authors Contribution
- Figure 1
- Figure 2
- Table 1
- Table 2
- Table 3
- Table 4
- Table 5
- Table 6
- References