Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/191
Expert Consensus | Volume 11 | Issue 3 Open Access
Consensus Summary on the Clinical Application of Patient-Controlled Analgesia for Pain Management in China
Shouzhang She1, Weifeng Yu2, Yuguang Huang3, Shanglong Yao4, Buwei Yu5, Changhong Miao6, Tianzuo Li7, Weidong Mi8, Jin Liu9, Tao Zhu9, Xiaoming Deng10, Jianjun Yang11, Qinjun Chu12, Cunming Liu13, Wenqi Huang14, Minghui Cao15, Ziqing Hei16, Bin Zheng1, Yanlu Ying1, Yan Luo5, Wen Ouyang17, Fan Su18, Le Shen3, Zhen Hua19, Mingjun Xu20, Junming Ye21, Xiaochun Zheng22, Xiuli Wang23, Xuebing Xu24, Jiaqiang Zhang25, Jianfeng Zhang26, Shibiao Chen27, Haihua Shu28, Xiaohong Chen29, Zhiping Wang30, Shaoshuang Wang31, Jie Xiao2, Hui Qiao7, Zhiheng Liu32, Heng Li33, Yi Feng34, Hanbing Wang35, Ye Zhang36, Yun Wang37, Yibin Qin38, Pingbo Xu39. Writing group of the Anesthesiology Branch of the Chinese Medical Association for expert consensus on clinical application practice guidelines in patient-controlled analgesia
1Medical Center of Anesthesia Quality Assurance of Guangzhou (Guangzhou First People's Hospital), China
2Department of Anesthesiology, Renji Hospital, Shanghai Jiao Tong University School of Medicine, China
3Department of Anesthesiology, Chinese Academy of Medical Sciences, Peking Union Medical College, China
4Department of Anesthesiology, Union Hospital, Tongji Medical College of Huazhong University of Science and Technology, China
5Department of Anesthesiology, Ruijin Hospital, Medical College of Shanghai Jiao Tong University, China
6Department of Anesthesiology, Zhongshan Hospital Affiliated to Fudan University, China
7Department of Anesthesiology, Beijing Shijitan Hospital, China
8Department of Anesthesiology of the First Medical Center, Chinese PLA General Hospital, China
9Department of Anesthesiology, West China Hospital of Sichuan University, China
10Department of Anesthesiology, Faculty of Anesthesiology, Changhai Hospital, China
11Department of Anesthesiology, The First Affiliated Hospital of Zhengzhou University Pain and Perioperative Medicine, China
12Department of Anesthesiology and Perioperative Medicine, Zhengzhou Central Hospital, China
13Department of Anesthesiology and Perioperative Medicine, First Affiliated Hospital of Nanjing Medical University, China
14Department of Anesthesiology, The First Affiliated Hospital, Sun Yat-sen University, China
15Department of Anesthesiology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou City, China
16Department of Anesthesiology, The Third Affiliated Hospital of Sun Yat-sen University, China
17Department of Anesthesiology, The Third Xiangya Hospital, Central South University, China
18Department of Anesthesiology, The Affiliated Hospital of Shandong University of Traditional Chinese Medicine, China
19Department of Anesthesiology, Beijing Hospital, China
20Department of Anesthesiology, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, China
21Department of Anesthesiology, First Clinical Medical College, Gannan Medical University, China
22Department of Anesthesiology, Fujian Provincial Hospital, China
23Department of Anesthesiology, The Third Hospital of Hebei Medical University, China
24Department of Anesthesiology, Shenzhen Hospital of Hongkong University, China
25Department of Anesthesiology and Perioperative Medicine, Henan Provincial People's Hospital, China
26Department of Anesthesiology, Yining County Traditional Chinese Medicine Hospital, Yining County, Ili Prefecture, Xinjiang Uygur Autonomous Region, China
27Department of Anesthesiology, The First Affiliated Hospital of Nanchang University, China
28Department of Anesthesiology, Guangdong Provincial People's Hospital (Guangdong Academy of Medical Sciences), China
29Department of Anesthesiology, Tumor Hospital Affiliated to Nantong University, China
30Department of Anesthesiology, Affiliated Hospital of Xuzhou Medical University, China
31Department of Anesthesiology, First Affiliated Hospital of Xi 'an Jiaotong University, China
32Department of Anesthesiology, The First Affiliated Hospital of Shenzhen University (Shenzhen Second People's Hospital), China
33Department of Anesthesiology, Affiliated Qingyuan Hospital of Guangzhou Medical University, China
34Department of Anesthesiology, Peking University People's Hospital, China
35Department of Anesthesiology, The First People's Hospital of Foshan City, China
36Department of Anesthesiology, The Second Affiliated Hospital of Anhui Medical University, China
37Department of Anesthesiology, Qinghai Provincial Hospital, China
38Department of Anesthesiology, Affiliated Hospital of Nantong University, China
39Department of Anesthesiology and Pain Rehabilitation, Zhejiang Cancer Hospital, China
She Shouzhang, Department of Anesthesiology, Guangzhou First People's Hospital, China, E-mail: sheshouzhang@163.com;Yu Weifeng, Department of Anesthesiology, Renji Hospital, Shanghai Jiao Tong University School of Medicine, China, E-mail: ywf808@yeah.net;
Huang Yuguang, Department of Anesthesiology, Chinese Academy of Medical Sciences, Peking Union Medical College, China, E-mail: garybeijing@163.com
Editor: Renyu Liu, MD; PhD; Professor, Department of Anesthesiology and Critical Care, Perelman School of Medicine at the University of Pennsylvania, Center of Penn Global Health Scholar, 336 John Morgan building, 3620 Hamilton Walk, Philadelphia, PA 19104, USA, Fax: 2153495078, E-mail: RenYu.Liu@pennmedicine.upenn.edu
Received: August 08, 2020 | Accepted: December 09, 2024 | Published: December 12, 2024
Citation: She SZ, Yu WF, Huang YG, Yao SL, Yu BW, et al. Consensus Summary on the Clinical Application of Patient-Controlled Analgesia for Pain Management in China; 11(3):635-652
Abstract
Patient-Controlled Analgesia (PCA) has been shown to alleviate neurological damage and inflammatory stress response, and reduce acute pain. The implementation of PCA requires specialized PCA pumps, which have undergone continual development and improvement over the past 30 years. With the rapid advancement of computer-network-intelligent technologies in clinical medicine, China has research and development an intelligent 8Analgesic pumps system, named Artificial Intelligence PCA (Ai-PCA). This system significantly enhances the precision, reliability, and safety of PCA pain management. However, challenges remain due to the significant individual differences in pain perception, the variety of analgesic drug administration schemes, and the less than satisfactory treatment satisfaction. Experts in anesthesiology and pain medicine have been invited to compile the “Consensus summary on the clinical application of patient-controlled analgesia for pain management in China;” to provide guidance for clinical physician.
Keywords
Analgesia patient-controlled, Pain, Postoperative, Standardization, Consensus
Introduction
The consensus, spearheaded by Guangzhou First People's Hospital and authored on behalf of the Expert Working Group of the Chinese Society of Anesthesiology, involved 46 experts nationwide who participated in voting via a Tencent QR code-based questionnaire system. The voting process was used to establish the strength of recommendations for the Expert Consensus on the Clinical Application of Patient-Controlled Analgesia. The results showed that 100% of the experts participated in the QR code-based voting, selecting either "agree" or "disagree." Three experts (6.5%) abstained by selecting "unknown" or "unsure" for certain options. However, after the voting, all experts (100%) agreed to endorse the results generated through this QR code-based voting process for the Expert Consensus on the Clinical Application of Patient-Controlled Analgesia. In 2020, the International Association for the Study of Pain (IASP) revised its definition of pain. The updated definition states: “Pain is an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage” [1]. Pain is inherently a subjective experience, influenced to varying degrees by biological, psychological, and social factors. Pain can be categorized into acute pain and chronic pain. Almost all surgical procedures result in tissue and nerve damage as well as inflammatory responses, which subsequently activate nociceptors and produce varying degrees of pain. Pain stress may lead to a series of disturbances and dysfunctions in respiratory, circulatory, endocrine, and metabolic functions, thereby affecting surgical outcomes and postoperative recovery [2]. Patient-controlled analgesia (PCA) is a technique in which healthcare providers preset the dosage of analgesic medication based on the patient's pain level and physical condition using a PCA device. This allows patients to manage their own pain, potentially alleviating stress and inflammatory responses caused by surgical trauma, thereby reducing perioperative discomfort and accelerating postoperative recovery [3-10].
Origin and Development of PCA
In the early 1970s, Sechzer introduced the principle of demand-based analgesia for PCA, which allows patients to self-administer analgesics by pressing a bolus button on a PCA pump, adjusted to their pain level and need, based on the medication prepared by healthcare providers. With the integration of computer technology and medicine, Grasbus produced the first PCA pump (The Cardiff Palliator) in 1976 [11].
The concept of PCA was introduced to mainland China in 1993, and electronic analgesia pumps were implemented in 1994. The PCA pump administers analgesics through a microcomputer-controlled infusion pump with a safety control system. Anesthesiologists pre-program the dosage and regimen, and patients can self-administer the medication by pressing a button when they experience pain. Over the past 30 years, the development of PCA pumps has evolved with technological advancements, and the introduction of intelligent PCA pumps, driven by computer technology, has significantly improved the precision, reliability, and safety of PCA treatment [12].
Definition and Advantages of PCA
Definition of PCA
PCA refers to a specialized micro-infusion device controlled by a computer, connected to the patient through tubing, which continuously delivers analgesics at a specified rate. The device is usually equipped with a self-controlled button, allowing patients to increase the dose when they experience heightened pain. Anesthesiologists adjust the continuous infusion rate, single bolus dose, and lockout time [13].
Types of PCA pumps
There are three commonly used PCA pumps in clinical practice: disposable mechanical infusion pumps, electronic programmable infusion pumps, and network-managed (smart) infusion pumps [14].
Advantages of PCA
PCA is primarily used in the management of acute and chronic pain, including postoperative pain, labor pain, cancer pain, and pain management for critically ill adult patients [15-18]. Following the principle of “pain relief on demand,” PCA addresses the analgesic needs of different patients at varying times and pain intensities, making it an effective tool to reduce the individual variability of pain management and increase patient satisfaction [19].
Consensus Development Methods and Basis
Relevant literature on PCA technology, clinical applications, and perioperative management were retrieved from PubMed, Embase, Web of Science, Chemical Abstracts Service (CAS), Wan fang Data, China National Knowledge Infrastructure (CNKI), Chinese Core Journals, Chinese Science Citation Database, and EBSCO Academic databases. The search period was from database inception to March 1, 2024. Using evidence-based medicine and combining years of clinical experience, the group consisting of experts from anesthesiology and pain medicine departments across the China organized by the Chinese Society of Anesthesiology engaged in multiple discussions and revisions to finalize this expert consensus.
Additionally, the numerical rating scale (NRS) [20], visual analogue scale (VAS) [21], and the grade of recommendations assessment, development, and evaluation (GRADE) tool for evidence grading [22] were used (Table 1). Combining existing national and international guidelines and expert consensus, the “Expert Consensus on the Standardized Clinical Application of Patient-Controlled Analgesia” was drafted, with evidence quality categorized into four levels: High (A), moderate (B), low (C), and very low (D). Recommendations were classified as strong or weak (Table 1), with consensus strength determined by expert voting via a Tencent scan code questionnaire (support = 100% for “strong consensus,” support ≥ 80% for “consensus,” support ≤ 60% for “no consensus,” and support = 0% for “rejection of recommendation”). This paper provides evaluations and recommendations regarding PCA drug regimens, clinical PCA settings, and adverse reaction management.
Table 1: Grading of evidence quality based on NRS/VAS and GRADE.
|
Items |
Specific description |
Study type |
|
Evidence quality grading |
|
|
|
High (A) |
Very confident that the true effect is close to the estimated effect. |
Randomized controlled trials (RCTs) |
|
Moderate (B) |
Moderately confident in the effect estimate; the true value is likely to be close to the estimate, but differences may exist. |
High-quality secondary observational studies Randomized controlled trials (RCTs) downgraded by one level |
|
Low (C) |
Limited confidence in the effect estimate; the true value may differ from the estimate. |
Observational studies upgraded by one level Randomized controlled trials (RCTs) downgraded by two levels |
|
Very Low (D) |
Little confidence in the effect estimate; the true value is likely to be substantially different from the estimate. |
Observational studies Randomized controlled trials (RCTs) downgraded by three levels |
|
Recommendation Strength |
|
|
|
Strong Recommendation |
NRS/VAS score 4–6: Moderate pain, affecting sleep but still possible to sleep; 7–10: Severe pain, preventing sleep. Clearly demonstrates that the benefits outweigh the risks or vice versa. |
- |
|
Weak Recommendation |
NRS/VAS score 0: No pain; 1-3: Mild pain, not affecting sleep; Benefits and risks are uncertain or evidence, regardless of quality, shows that benefits and risks are approximately equal. |
- |
PCA Analgesic Medications
Opioids
Postoperative PCA often requires opioid medications, including the fentanyl class, such as fentanyl, sufentanil, remifentanil, and alfentanil; other opioid analgesics include morphine, hydromorphone, dezocine, oxycodone, pentazocine, butorphanol, buprenorphine, and nalbuphine [23-27].
Non-steroidal anti-inflammatory drugs (NSAIDs)
NSAIDs, which provide analgesic and antipyretic effects, are commonly used according to the patient's analgesic needs. Non-selective COX inhibitors such as flurbiprofen axetil injection are commonly used, and continuous PCA infusion is currently possible with flurbiprofen axetil [28,29]. Ketorolac injections can also be placed in the PCA pump for patient-controlled intravenous analgesia (PCIA) [30].
Local anesthetics
Long-acting local anesthetics commonly used include ropivacaine, bupivacaine, levobupivacaine, and liposomal bupivacaine. Short-acting local anesthetics such as lidocaine are also used. Epidural multimodal analgesia typically requires combining local anesthetics with opioids. For peripheral nerve blocks, local anesthetics are generally used without an opioid combination [31-36].
Adjuvant analgesics
Common adjuvant analgesics for PCA include dexmedetomidine, esketamine, dexamethasone, ondansetron, and droperidol, which can be selected for clinical use; however, antiemetics are not recommended for inclusion in the PCA pump [37-39]. Other adjunctive medications include acetaminophen, duloxetine, gabapentin, and pregabalin, which must be administered orally and not via PCA [40-42]. The characteristics of commonly used opioid analgesics are detailed in Table 2 [43].
Table 2: Characteristics of commonly used opioid analgesics in clinical practice.
|
Opioid |
Onset time (min) |
Elimination half-life (h) |
Plasma protein binding rate |
Pharmacological action |
|
Morphine |
5~10 |
3~4 |
26%~36% |
A pure opioid receptor agonist, morphine activates μ, κ, and δ receptors, leading to analgesia, respiratory depression, euphoria, and addiction. |
|
Fentanyl |
1~2 |
2~4 |
80% |
A μ-opioid receptor agonist, primarily metabolized in the liver with a significant first-pass effect. |
|
Sufentanil |
1~3 |
13 |
91%~93% |
A highly selective μ-opioid receptor agonist, with 7-10 times higher affinity for μ-receptors than fentanyl, offering good hemodynamic stability. |
|
Hydromorphone |
1~3 |
2~3 |
8%~19% |
A full opioid agonist with relative selectivity for μ-opioid receptors, without the analgesic ceiling effect seen with morphine. |
|
Butorphanol |
3~5 |
3~4 |
80% |
Acts as both an agonist and antagonist at μ receptors, with its primary metabolite activating κ receptors, interacting with CNS receptors for analgesia. |
|
Oxycodone |
2~3 |
3.5 |
45% |
A dual agonist at μ and κ receptors, with relative selectivity for μ receptors but can bind to other opioid receptors at higher doses. |
Common Clinical Applications of PCA
PCIA
Common opioid analgesics for PCIA: PCIA typically involves the use of potent opioids, supplemented by NSAIDs, ketamine, and antiemetics. Commonly used analgesics exhibit the following relative potency: Morphine 10 mg ≈ fentanyl 0.1 mg ≈ sufentanil 0.01 mg ≈ hydromorphone 1 mg ≈ tramadol 100 mg ≈ pethidine 100 mg ≈ butorphanol 2 mg ≈ oxycodone 10 mg ≈ dezocine 10 mg. The pharmacokinetics and pharmacodynamics of different PCIA drugs determine variations in parameters such as single dose and lockout time [18,20,23,24,27,44-50]. Postoperative PCIA can employ multimodal approaches, including pre-emptive analgesia and preventive analgesia [51]. These approaches align with the concept of ERAS, promoting the development of traditional PCA [52].
Indications for PCIA: PCIA is indicated for moderate to severe postoperative pain, particularly in patients unable to tolerate oral medications. It is suitable for analgesia in all body regions and is appropriate for adults with ASA I-III classifications and pediatric patients (age ≥ 6 months), provided there is no preoperative cough, expectoration, or significant abnormalities in cardiac, pulmonary, hepatic, or renal function. PCIA can also be used for the maintenance of severe cancer pain, management of refractory cancer pain, and effective control of breakthrough pain [18,20,23,24,27,44-50].
Absolute contraindications for PCIA: (1) Coma or altered consciousness; (2) Cognitive impairment preventing proper understanding and use of PCA technology; (3) Severe obstructive sleep apnea syndrome; (4) Patient refusal [17,53].
Relative contraindications for PCIA: (1) Systemic infection, heart or lung failure, coagulopathy, or severe hepatic and renal insufficiency; (2) Acute upper gastrointestinal bleeding or obstruction leading to a risk of reflux and aspiration; (3) Conscious patients unable to operate the "self-control" button due to physical limitations; (4) Psychiatric disorders, including sleep apnea [54].
Advantages of PCIA: PCIA offers broad indications, is applicable to adults and children, and can be used for analgesia across all body regions. Its clinical use is widespread, often involving potent μ-opioid receptor agonists, partial agonists, or agonist-antagonists [54]. Due to the high toxicity and addiction potential of pethidine, as well as its resistance to naloxone, pethidine is not recommended for analgesia in PCIA patients [55].
Disadvantages of PCIA: There is considerable variability in analgesic efficacy among individuals, and increasing PCIA drug dosages is associated with an increased risk of side effects such as nausea, vomiting, dizziness, pruritus, excessive sedation, respiratory depression, and hypotension.
Assessment of PCIA analgesic efficacy: Assessment can be performed using the NRS, VAS, Verbal Rating Scale (VRS), Wong-Baker Faces Pain Rating Scale, and Bruggrmann Comfort Scale (BCS) for comfort level, and Ramsay Sedation Score for sedation level. Other commonly used tools include the McGill Pain Questionnaire (MPQ) and the analgesic quality index (AQI) for Ai-PCA systems. Side effects such as nausea, vomiting, pruritus, and dizziness are monitored and recorded during routine follow-up assessments.
Epidural PCA (PCEA)
Anesthetic drugs for PCEA: Epidural PCA typically requires a combination of opioids and long-acting local anesthetics for analgesia. The use of local anesthetics and opioids in the epidural space provides a synergistic analgesic effect, allowing for the reduction of local anesthetic concentrations and opioid dosages. Opioids directly act on opioid receptors in the spinal cord, producing analgesic effects, which, compared to intravenous and oral administration, reduces the risk of opioid-related side effects and potential complications [20,56-58]. PCEA can offer effective and prolonged segmental analgesia [59,60].
Indications for PCEA: (1) All patients undergoing epidural anesthesia can receive postoperative PCEA. Guidelines recommend PCEA after thoracic and abdominal surgeries; (2) Trauma patients, including those not requiring surgery, such as patients with rib fractures, can benefit from epidural analgesia, which alleviates pain during respiratory movements and may reduce the incidence of atelectasis and pulmonary inflammation; (3) Patients with pain syndromes [61].
Contraindications for epidural PCA: (1) Patient refusal; (2) Patients with coagulopathy or those currently undergoing or about to receive anticoagulant therapy; (3) Patients with bacteremia or local infection at the epidural puncture site; (4) Patients with altered consciousness or psychiatric disorders; (5) Patients with spinal deformities or spinal cord disorders (relative contraindication); (6) Patients with increased intracranial pressure or central nervous system diseases; (7) Patients in shock with severely compromised cardiovascular function; (8) Lack of qualified acute pain service personnel.
Advantages of PCEA: (1) Facilitates early mobilization; (2) Reduces pulmonary complications; (3) Decreases the incidence of deep vein thrombosis; (4) Shortens the recovery time of gastrointestinal function; (5) Reduces stress response and the occurrence of myocardial ischemia caused by pain; (6) Promotes graft survival after lower limb vascular surgery; (7) Reduces bladder spasms after prostate and hypospadias surgeries; (8) Use of spiral-reinforced wire catheters during epidural puncture helps reduce catheter breakage and the risk of nerve injury [62].
Disadvantages of PCEA: Some adverse effects and complications related to PCEA are associated with epidural puncture and catheter placement, such as epidural hematoma, spinal canal infection, and post-dural puncture headache. Others are related to the use of analgesic solutions (opioids and local anesthetics) [32]. With the increasing use of minimally invasive surgeries, which are associated with reduced pain intensity, PCEA has not become widely adopted for thoracic and abdominal surgeries. Recent literature suggests that PCEA prolongs hospital stays for thoracic surgery patients, whereas abdominal surgery patients benefit more from PCEA [63].
Assessment of PCEA analgesic efficacy: Pain assessment for PCEA follows the same objective methods as for PCIA [34]. In addition, the modified Bromage scale is used to evaluate the degree of motor block, while the Frankel grading system assesses the extent of spinal cord injury. Patient comfort with analgesia is evaluated using the AQI [36,37].
Other PCA administration routes
Subcutaneous PCA (PCSA): PCSA involves the insertion of a fine catheter under the skin at a designated site, allowing for the administration of opioids through a PCA pump. The absorption of the drug through subcutaneous tissue occurs slowly, resulting in a delayed onset of analgesia. Despite this drawback, PCSA offers several advantages, including enhanced safety and minimal side effects. However, the primary limitation remains the slow onset of pain relief.
Subarachnoid PCA (S-PCA): S-PCA involves the placement of a specially designed catheter directly into the subarachnoid space following a spinal puncture to facilitate analgesic delivery [9]. There are two forms of S-PCA: (1) A dedicated catheter can remain in the subarachnoid space for up to 48 hours, making it suitable for pain management in the lower limbs or abdomen. Exceeding this duration may lead to spinal cord irritation and hyperthermia, both of which resolve upon catheter removal. (2) A novel catheter made from thermoplastic polyurethane (TPU) can be placed in the subarachnoid space, with the drug reservoir implanted subcutaneously. The PCA pump delivers medication via a needle that punctures the reservoir, and in CP mode, this catheter can remain in place for 1 to 3 years, making it ideal for long-term pain management in cancer patients. The primary advantage of S-PCA is its rapid onset and potent analgesic effect; however, the major drawback is the elevated risk of severe infection. Therefore, strict aseptic techniques are essential throughout the procedure to minimize infection risk.
PCEA following subarachnoid injection: In the context of combined spinal-epidural anesthesia, the dura mater is punctured, creating a small opening that allows medication to seep into the subarachnoid space. Under these circumstances, PCEA remains a safe and effective option [64,65].
Target-controlled infusion PCA (TCI-PCA): Target-controlled infusion (TCI), when integrated with PCA, is referred to as TCI-PCA. For example, when a patient activates the bolus button for remifentanil, an intelligent intravenous infusion system semi-automatically adjusts the target drug concentration, swiftly achieving the desired plasma or effect-site concentration. The primary advantage of TCI-PCA is its rapid onset; however, the duration of analgesia tends to be relatively short. Studies have demonstrated the effectiveness of intravenous remifentanil TCI-PCA for labor analgesia [66].
Peripheral nerve block PCA (PNB-PCA): Ultrasound-guided PNB or continuous peripheral nerve block (CPNB) involves the administration of local anesthetic solutions through intermittent injections or continuous catheter infusion for PCNA. This approach is effective not only for managing pain in major surgeries of the upper and lower limbs but also for providing perioperative analgesia in patients undergoing abdominal, plastic, urological, gynecological, thoracic, and trauma surgeries [67].
Multimodal analgesia combined with PCA:
(1) PCA administration modes
1) LCP mode: Loading dose + continuous dose + PCA (LCP for short), where an initial loading dose is followed by continuous dosing, with the patient pressing the PCA button when pain is experienced. 2) CP mode : Continuous dosing + PCA, where a baseline dose of the drug is continuously delivered, and the patient presses the bolus button when experiencing pain. 3) P mode : Pure PCA self-control throughout the analgesic period, where the patient presses the PCA button when pain occurs. The use of a loading dose helps maintain the minimum effective analgesic concentration (MEAC) required by the patient. With an appropriately chosen loading and continuous dose, the plasma concentration is more easily maintained within MEAC across different age groups without overdose. When PCA is configured using equivalent doses, there is no statistical difference in analgesic efficacy between long-acting opioid PCA without background dosing and the LCP mode, though the duration differs. Drug selection should be based on the extent of surgical trauma to ensure standardized management [68].
(2) PNB + PCIA multimodal administration
Long-acting local anesthetics such as ropivacaine can be used for PNB either preoperatively or postoperatively in the PNB+PCIA mode. This approach significantly reduces the need for analgesic medications. Using ultrasound-guided liposomal bupivacaine for PNB provides more complete and long-lasting postoperative analgesia, facilitating early functional rehabilitation and contributing to rapid postoperative recovery [69].
Pre-PCA Assessment and Patient-Family Education
Preoperative assessment
The safe and effective management of Patient-Controlled Analgesia (PCA) requires an interdisciplinary Acute Pain Service (APS) team composed of physicians, pharmacists, and nurses. Before initiating PCA, the physician should assess the patient's American Society of Anesthesiologists (ASA) classification, body mass index (BMI), type of surgery, and blood transfusion status and accurately evaluate postoperative pain using the NRS. Additionally, the physician must select the appropriate analgesic pump and consider factors that may influence the effectiveness of postoperative PCA. The physician provides orders, the nurse implements them, and the APS ensures accurate medication preparation, continuous assessment of analgesic efficacy, and dynamic monitoring for adverse reactions. Team members must be well-versed in the PCA management process, including patient selection, comprehensive identification and assessment of high-risk patients, formulation of PCA medication regimens, dose adjustments during use, and monitoring and management of adverse effects. Effective communication among team members is essential to achieve optimal pain control outcomes and prevent complications [70,71].
Preoperative education
For cognitively alert patients who can comprehend the information provided by healthcare professionals, preoperative education is critical. Repeated instruction can enhance patients' understanding and retention of information. The educational content should include (1) The importance of postoperative pain relief, (2) The principles and safety of the PCA pump, and (3) Key points regarding its use. Proper education by the physician is crucial for both patients and family members to ensure the safe use of PCA. Before initiating PCA analgesia, it is essential to communicate with the patient and their family, providing a detailed explanation of the advantages and safe use of the PCA pump tailored to their varying occupations and educational backgrounds. Training should emphasize the correct use of the device and clearly state that neither the patient nor family members should adjust PCA parameters independently. Specifically, only the patient or an authorized individual should press the PCA button to prevent accidental harm [72].
Structure of Artificial Intelligence PCA (Ai-PCA)
Ai-PCA system is a pain management pump that integrates the Internet of Things (IoT) and artificial intelligence (AI) technologies. It was approved for registration by the National Medical Products Administration in May 2011. The Ai-PCA consists of an intelligent analgesia terminal (including an intelligent infusion driving device and a disposable drug reservoir), a base station (used for data transmission), and a central analgesia monitoring station (equipped with analgesia management software on a computer, tablet, or smartphone) [73].
Base station
The base station serves as the foundational equipment for network setup, data reception, and transmission. It is equipped with a wireless module, an RS232 serial port at the bottom, a rubber antenna on the right side of the case, and status indicator lights on the front panel. The base station is a plug-and-play device. It acts as a key unit in the analgesia information system communication network, collecting data from the intelligent analgesia terminal and transmitting it to the central analgesia monitoring station [74].
Central analgesia monitoring station
The central monitoring station is responsible for analyzing and processing the data uploaded from the analgesia terminal. Operators can monitor the use of the analgesia terminal through the central station, which automatically generates postoperative follow-up records and PCA documentation. Hospitals are required to comply with the announcement issued by the National Medical Products Administration (CFDA) regarding the "Medical Device Classification Catalog" (No. 104 of 2017). Departments must also adhere to the national requirement that the "Analgesia Infusion Information Collection System" be registered as a Class III medical device [75].
PCA Standardized Management System
PCA standardized operational management
The anesthesia department implements the management model of a "Cloud Ward" or "Virtual Pain Unit (VPU)," adhering to a system of full participation, comprehensive control, and overall quality assurance [76]. Physicians from the APS are responsible for preoperative visits, where they inform patients about pain management strategies [77]. In compliance with the "Notice on Strengthening the Management of Narcotic Drugs and Category I Psychotropic Substances in Medical Institutions" (National Health Medical Issue 2020-13), anesthesiologists are not permitted to handle drugs independently. According to the "Law of the People's Republic of China on Practicing Physicians," the anesthesia department must establish an order-based system, with APS physicians issuing electronic orders. Nurses are responsible for preparing analgesic medications under supervision, and infusion pump parameters are automatically entered through the smart order system to enhance efficiency and prevent manual entry errors [78]. Due to the complexity and diversity of PCA formulations, a dual-verification system should be used in clinical practice to strengthen the Analgesia Quality Index (AQI) and improve the overall effectiveness of PCA. The workflow for Ai-PCA management is shown in Figure 1 [79].
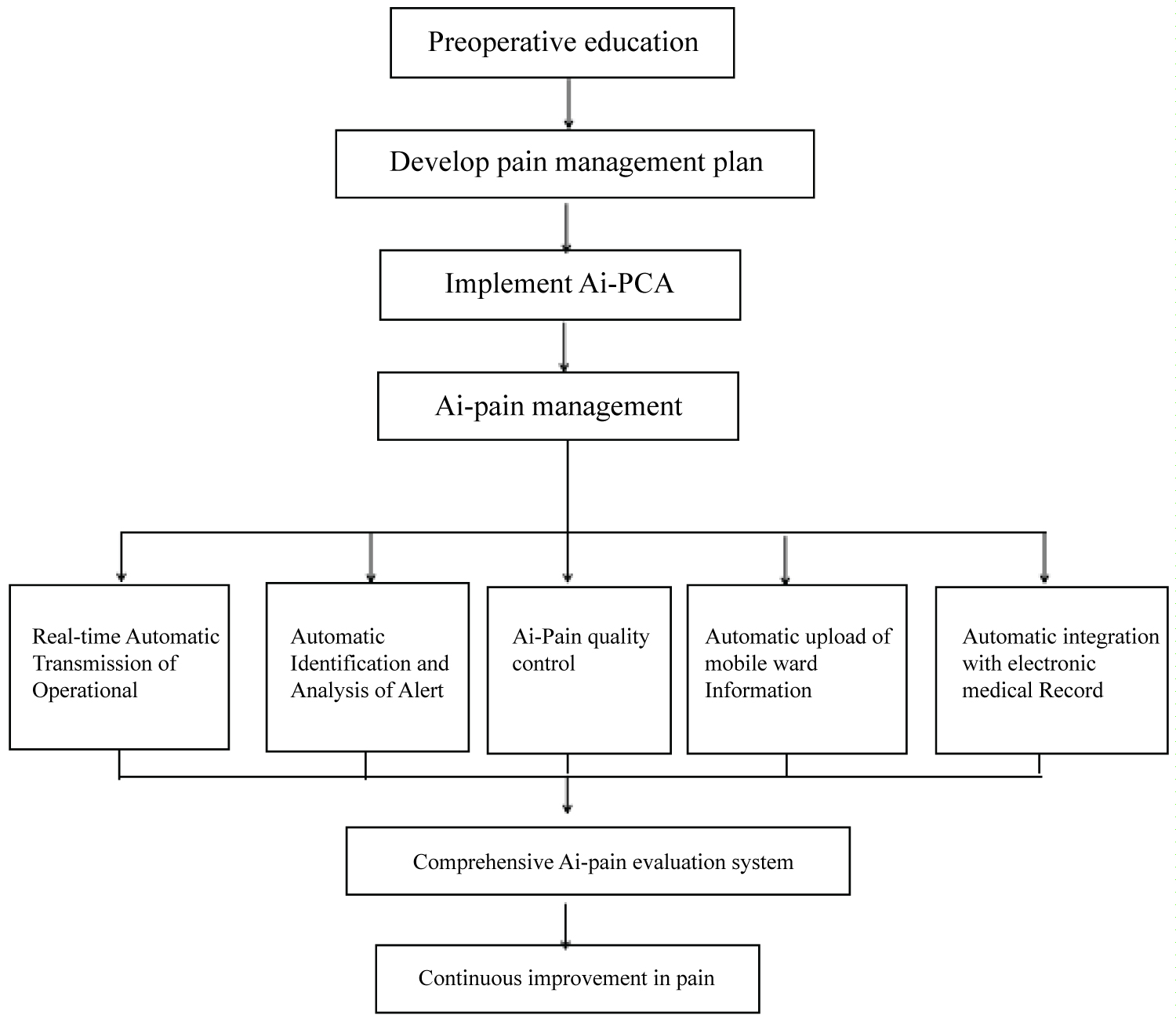
Figure 1:
Preoperative education Develop pain management plan Implement Ai-PCA Ai-pain management Real-time Automatic Transmission of Operational Automatic Identification and Analysis of Alert Ai-Pain quality control Automatic upload of mobile ward Information Automatic integration with electronic medical Record Comprehensive Ai-pain evaluation system Continuous improvement in pain
APS operates 24 hours a day, 7 days a week, ensuring that all patients receiving pain treatment are managed by the on-duty APS physician, who addresses alarms and other issues as they arise. The APS team maintains dedicated application forms, registration logs, and routine nursing records. APS physicians conduct daily rounds 1 to 3 times, during which they assess VAS scores, Behavioral Comfort Scale (BCS) comfort scores, sedation levels, monitor SpO 2 , and check the functionality of PCA pumps. Additionally, anesthesiologists and nurses perform afternoon rounds to evaluate the analgesic effects of PCA, identify any adverse reactions, and respond to questions regarding pain management. A VAS score of ≤ 3 indicates effective analgesia, while a score of ≥ 4 and/or the presence of adverse reactions necessitates timely symptomatic treatment, contributing to Enhanced Recovery After Surgery (ERAS) [80]. A three-level quality control management model alongside the anesthesia department management workflow is illustrated in Figure 2.
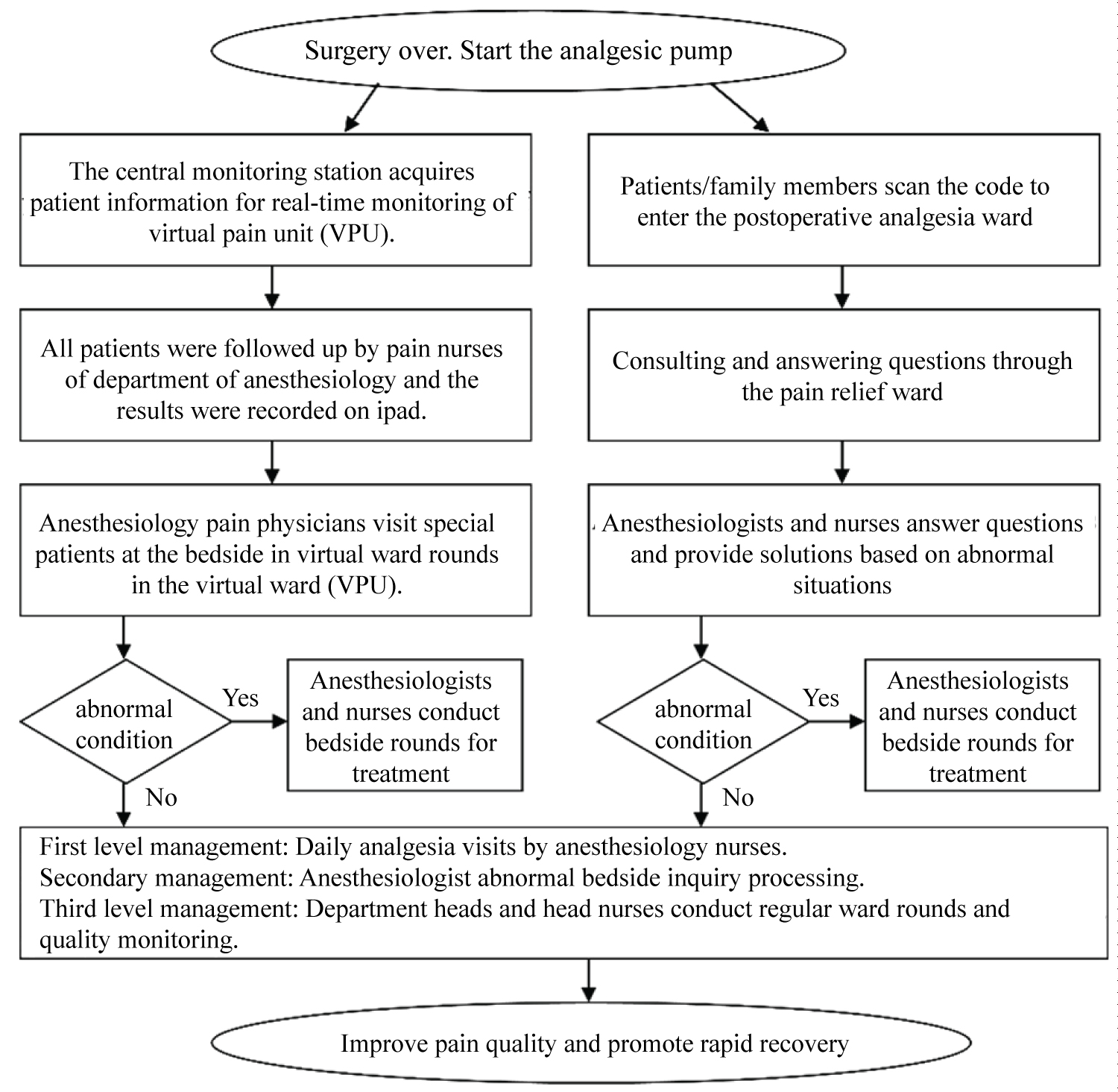
Figure 2:
Surgery over. Start the analgesic pump ➢ The central monitoring station acquires patient information for real-time monitoring of virtual pain unit (VPU).
➢ All patients were followed up by pain nurses of department of anesthesiology and the results were recorded on ipad. ➢ Anesthesiology pain physicians visit special patients at the bedside in virtual ward rounds in the virtual ward (VPU).
• Patients/family members scan the code to enter the postoperative analgesia ward • Consulting and answering questions through the pain relief ward
• Anesthesiologists and nurses answer questions and provide solutions based on abnormal situations Abnormal condition Yes - Anesthesiologists and nurses conduct bedside rounds for treatment No - First level management: Daily analgesia visits by anesthesiology nurses. Secondary management: Anesthesiologist abnormal bedside inquiry processing. Third level management: Department heads and head nurses conduct regular ward rounds and quality monitoring. • Improve pain quality and promote rapid recovery
Quality control management of the intelligent analgesia system
An integrated platform for multi-modal evaluation, recording, and querying has been established, enabling real-time remote management and monitoring of the analgesia terminal. After the APS nurse removes the catheter and discontinues the pump, the intelligent analgesia pump is placed into an intelligent residual volume disposal robot, which automatically registers, disposes of, and disinfects the residual medication [81].
Standardization of PCA data collection: Postoperative follow-up and patient PCA records are an essential part of the surgical patient’s medical record system. During the patient’s pain management process, medical data is collected from the source in a standardized format to ensure comprehensive and accurate data capture. This guarantees the consistency of data collection, storage, processing, analysis, extraction, and application.
Standardization of real-time PCA tracking: The system automatically collects postoperative pain management information from patients and generates medical records. This enables quality control and real-time tracking throughout the entire pain management process. By objectively recording changes in the patient’s condition in real time, it reduces the risk of missing important information during busy periods, thus aiding in accurate analysis and decision-making. Additionally, it prevents errors that may arise when physicians retrospectively fill in the records, thereby improving overall efficiency.
Digitalization of PCA information storage: The system utilizes computer database storage technology to record, process, and store information related to the patient’s pain management. The intelligent analgesia system allows on-demand retrieval of relevant medical data, which can be transferred to data mining software such as SPSS or SAS for macro-level analysis and scientific research. This provides strong support for managing medical practices related to patient analgesia, enabling the reproduction of PCA processes and the analysis of analgesic outcomes.
Intelligent quality control of PCA: The anesthesia department follows a pain management model characterized by full participation, continuous control, and comprehensive quality assurance. Using AQI software, the system analyzes key metrics such as the frequency of PCA button presses, evaluation rates, occurrence rates of various alarms, response times to critical alarms, medication utilization rates, and completeness of patient information. These indicators comprehensively reflect the technical proficiency of the medical staff, the thoroughness of patient assessments, the accuracy of medical orders, and the standardization of management practices. Departments typically select a 24-hour AQI display to provide a visual reflection of analgesic quality, and the PDCA (Plan-Do-Check-Act) cycle is used to continuously improve issues identified by the AQI. Additionally, comparative analyses of AQI data from different departments and hospitals help assess the quality of pain management and promote ongoing improvements in care [82].
Typical PCA Formulations
Recommended PCIA methods for postoperative patients
Recommended opioids for PCIA [83-85]: Morphine, sufentanil, and hydromorphone are the recommended strong opioids for PCIA (evidence level: B; recommendation grade: strong; consensus rate: 100.0%).
μ-Receptor partial agonist-antagonist for PCIA [87-89]: The use of μ-receptor partial agonist-antagonist drugs for PCIA is recommended (evidence level: B; recommendation grade: strong; consensus rate: 80%).
Fentanyl for PCIA [90]: Fentanyl is recommended for PCIA (evidence level: C; recommendation grade: weak; consensus rate: 37.2%).
Oxycodone for PCIA [49,91,92]: Oxycodone is recommended for PCIA (evidence level: C; recommendation grade: weak; consensus rate: 60.0%).
NSAIDs for PCIA [93]: The use of flurbiprofen axetil as an adjunctive analgesic during PCIA is recommended (evidence level: B; recommendation grade: strong; consensus rate: 86%).
Adjuvant analgesic medications for PCIA [94,95]: Dexmedetomidine and esketamine are recommended as adjuvant analgesic drugs during PCIA (evidence level: B; recommendation grade: strong; consensus rate: 97.7%) [4,19,23,49,56,57,81-110].
Combination of adjuvant drugs for PCIA [4,23]: Dexamethasone, droperidol, ondansetron, azasetron, and tropisetron are recommended as adjuvant drugs during PCIA to prevent opioid-induced nausea and vomiting (evidence level: B; recommendation grade: strong; consensus rate: 93.0%).
Other adjuvant drugs for PCIA [96]: Gabapentin and pregabalin are recommended as adjuvant medications during PCIA (evidence level: C; recommendation grade: weak; consensus rate: 16.3%).
Recommended PCEA methods for postoperative patients
Single-pump PCEA [56,57,97-109]: In the Single-pump PCEA model, strong opioids are administered using an LCP mode. The loading dose for titration is set at 5 ml per dose, with a continuous infusion of 0.5 ml per hour and a PCA bolus dose of 1 ml per activation, featuring a lockout interval of 10 to 15 minutes. The maximum allowable safety dose within one hour is capped at 15 ml. Strong opioids such as sufentanil, hydromorphone, and long-acting local anesthetics, including 0.2% ropivacaine and 0.2% levobupivacaine, are recommended for PCEA (evidence level: B; recommendation grade: strong; consensus rate: 95.3%).
Dual-pump PCEA: The dual-pump method is recommended internationally. Pump A continuously infuses 0.2% ropivacaine at 8-12 ml/h. When the analgesic effect of the local anesthetic is insufficient, Pump B is connected via a three-way valve to a venous catheter for 0.1% morphine PCIA [110-112]. Domestically, a dual-pump method is recommended where Pump A infuses 0.2% ropivacaine at 4-6 ml/h continuously. If the analgesia from the local anesthetic is insufficient, the patient can press the control button, and Pump B delivers 0.01% morphine PCEA via an epidural catheter. The LP model is used, with a loading (titration) dose of 5 ml/dose and a PCA bolus dose of 1-2 ml/dose, with a lockout interval of 15-20 minutes, enhancing analgesic effects [61,113,114]. However, when the local anesthetic exceeds 8 ml/h for more than 6 hours, patients often experience significant numbness in both legs. Patients have reported that this numbness is more uncomfortable than pain, prompting a request to stop the pump. Therefore, the dosage recommended domestically is significantly lower than that used internationally. Dual-pump analgesia is recommended for postoperative patients (evidence level: C; NRS/VAS score of 4-10: Weak; consensus rate: 60%).
PNB + PCEA [115-117]: The combination of PNB and PCEA is recommended for postoperative analgesia in patients (evidence level: B; NRS/VAS score of 4-10; recommendation grade: strong; consensus rate: 95.3%).
Recommended approaches for labor analgesia
PCIA for labor analgesia [118]: PCIA is recommended for labor analgesia in parturients (evidence level: C; NRS/VAS score of 4-10: Recommendation grade: weak; consensus rate: 16.3%).
PCEA for labor analgesia [119]: PCEA is strongly recommended for labor analgesia in parturients (evidence level: A; NRS/VAS score of 6-10: Recommendation grade: strong; consensus rate: 100%). cancer pain.
TCI-PCA for labor analgesia [120]: TCI-PCA is recommended for labor analgesia in parturients (evidence level: C; NRS/VAS score of 4-10: Recommendation grade: Weak; consensus rate: 18.6%).
Recommended approaches for angina pectoris
12.4.1. PCIA for angina pectoris [47]: PCIA is recommended for patients with angina pectoris (evidence level: B; NRS/VAS score of 4-10: Recommendation grade: weak; consensus rate: 74.4%).
PCEA for angina pectoris [121,122]: PCEA (T5-6) is recommended for patients with angina pectoris to significantly relieve paroxysmal chest pain or discomfort caused by myocardial ischemia due to insufficient coronary blood supply (evidence level: B; NRS/VAS score of 6-10: Recommendation grade: Strong; Consensus rate: 86.0%).
Recommended approaches for cancer pain
PCIA for moderate cancer pain [19,109,110,123-125]: PCIA is strongly recommended for moderate cancer pain using an on-demand administration model (evidence level: B; recommendation grade: strong; consensus rate: 95.3%).
PCIA with pethidine [126]: Pethidine is not recommended for PCIA in cancer pain management (evidence level: D; recommendation grade: not recommended; consensus rate: 0%).
PCEA for severe cancer pain [127]: For severe cancer pain, a multidimensional evaluation should be conducted before starting PCA. After titration with a strong μ-opioid receptor agonist, patient consent should be obtained, and treatment should begin with a low dose. Efficacy and adverse reactions should be dynamically assessed during titration, and parameters should be adjusted accordingly (evidence level: B; recommendation grade: strong; consensus rate: 86%).
S-PCA for refractory cancer pain [128]: For patients with severe refractory cancer pain in the lower abdomen and lower limbs, especially those unable to lie flat or with intense pain and a strong desire for relief, S-PCA with long-acting local anesthetics may be used after obtaining informed consent (evidence level: C; recommendation grade: strong; consensus rate: 60.5%).
PCSA for moderate refractory cancer pain [129]: PCSA with strong μ-opioid receptor agonists is recommended for patients with moderate refractory cancer pain (evidence level: C; NRS/VAS score of 4-10: recommendation grade: weak; consensus rate: 58.1%).
PNB + PCIA for surgical patients [130,131]: PNB combined with PCIA is recommended for appropriate surgical patients (evidence level: B; NRS/VAS score of 4-10: Recommendation grade: strong; consensus rate: 97.7%).
Recommended approaches for severe pain management in adult critical care patients
PCIA for severe chest pain in acute myocardial infarction (AMI) patients [24]: PCIA is recommended for adult critical care patients experiencing severe chest pain during an AMI (evidence level: B; NRS/VAS score of 4-10: Recommendation grade: weak; consensus rate: 60.5%).
PCIA with NSAIDs for severe chest pain in AMI patients [132]: The use of NSAIDs for pain relief in adult critical care patients with severe chest pain due to AMI is recommended (evidence level: C; NRS/VAS score of 4-10: Recommendation grade: weak; consensus rate: 16.3%).
PCIA with morphine for shock patients [133]: Morphine is recommended for pain relief in patients suffering from shock (evidence level: C; NRS/VAS score of 4-10: Recommendation grade: weak; consensus rate: 32.6%).
PCIA for patients with acute abdomen [134]: On-demand analgesia is strongly recommended for patients with moderate to severe pain and a clear diagnosis of acute abdomen (evidence level: B; NRS/VAS score of 4-10: Recommendation grade: strong; consensus rate: 95.3%).
PCEA for patients with acute abdomen [135]: PCEA is recommended for pain relief in patients with severe pain and a clear diagnosis of acute abdomen (evidence level: B; NRS/VAS score of 4-10: Recommendation grade: strong; consensus rate: 81.4%).
PCEA for acute severe pancreatitis [136]: Opioids are recommended for pain relief in patients with acute severe pancreatitis (evidence level: B; NRS/VAS score of 4–10: recommendation grade: strong; consensus rate: 90.7%).
PCIA/PCEA for postoperative severe pain [137,138]: Opioids are routinely recommended for postoperative pain management in severe pain cases (evidence level: B; NRS/VAS score of 4-10: Recommendation grade: strong; consensus rate: 97.7%).
PCIA for post-craniotomy pain [139,140]: PCIA is recommended for pain management in patients after cranial surgery (evidence level: C; NRS/VAS score of 4-10: recommendation grade: weak; consensus rate: 62.8%).
Considerations for PCA formulations
Before selecting multimodal analgesia drugs for postoperative PCA, a thorough assessment of the patient’s pain is required. Due to variations in patient ASA scores, types of surgery, and the nature of postoperative pain, the pharmacological effects of the drugs, combination regimens, PCA pump types, analgesic routes, and administration modes will differ. Therefore, PCA outcomes vary, and a standardized pain management approach is not advisable. Treatment should be tailored to individual patients and local circumstances, with objective evaluation and accurate determination of multimodal pain management strategies [20,27].
Monitoring and Management of Adverse Reactions to PCA
Adverse reactions and monitoring of PCIA
Adverse reactions of PCIA: Postoperative PCA adverse reactions can significantly impact patient satisfaction. The adverse reactions of PCIA are primarily associated with the side effects of analgesic drugs, including excessive sedation, respiratory depression, nausea and vomiting, constipation, and, in some cases, pruritus and urinary retention [23]. PCIA optimizes opioid administration by minimizing the pharmacokinetic and pharmacodynamic variability between individuals. Respiratory rate is a routine parameter for monitoring respiratory depression, but SpO 2 monitoring should be utilized whenever possible.
Adverse reactions of PCEA: Epidural analgesia may cause complications and adverse reactions, some of which are related to epidural puncture and catheter placement. Adverse reactions include respiratory depression, excessive sedation, hypotension, nerve injury, unilateral lower limb numbness with weakness, or lower limb motor dysfunction. Other adverse reactions may include postoperative nausea and vomiting, pruritus, drowsiness, dizziness, and urinary retention [141,142].
Device or operation-related adverse reactions of PCA: Adverse reactions due to improper puncture technique or equipment use, such as bleeding or infection, are rare. Errors in pump programming, device malfunction, tampering with parameters, or family members pressing the PCA button on behalf of the patient may lead to drug overdose, resulting in serious adverse reactions like respiratory depression. Therefore, patients should be closely monitored for vital signs, particularly respiratory depression and changes in consciousness, during the first 24 hours of PCA initiation or after any dosage adjustment [143].
Adverse reactions of ultrasound-guided PNB + PCA: Ultrasound-guided PNB is a safe and effective postoperative analgesic technique. However, nerve blocks still carry risks of bleeding, infection, and nerve injury [20,27].
Management of adverse reactions
Respiratory depression: Respiratory depression is the most life-threatening adverse reaction. If the respiratory rate drops below 8 breaths/min, the patient's status, skin color, and airway patency should be immediately checked. For drowsy patients, respiratory patterns should be observed, and 0.1-0.4 mg of naloxone should be administered intravenously. In cases of mild airway obstruction, where the patient is easily aroused, they should be encouraged to choose the most suitable position to maintain airway patency [144].
Shivering: Postoperative muscle shivering may occur due to the side effects of morphine. Local application of a hot water bottle should be avoided. It is important to differentiate shivering from postoperative fever or infusion reactions and monitor whether the shivering is followed by an increase in body temperature [145].
Lower limb numbness with weakness: Lower limb numbness accompanied by weakness is a potential side effect of local anesthetics. This condition may arise from the displacement of the epidural catheter tip toward the nerve roots, excessive dosages of analgesics, or unilateral diffusion of the local anesthetic. Slowing the infusion rate can help alleviate these symptoms [146]. Typically, limb numbness resolves quickly after catheter removal and does not require special treatment. It is essential to implement precautionary measures and provide patient education to prevent falls during the use of the analgesic pump.
Urinary retention: Urinary retention is a common adverse reaction of opioid analgesics. Most patients using PCA pumps postoperatively are catheterized, and it is recommended that catheter removal should be timed after discontinuation of the analgesic pump [147].
Drowsiness: Some patients using PCA pumps may experience drowsiness. Patients should be easily roused, and nurses should frequently wake the patient, closely monitoring their respiratory rate, rhythm, depth, skin, lips, and nail bed color. Anesthetists should be informed to determine whether the analgesic dosage needs to be reduced [148]. During periods of drowsiness, nurses should enhance monitoring, raise bed rails, and educate caregivers to ensure patient safety, preventing falls, accidental catheter removal, or burns.
Hypotension: Hypotension may be associated with changes in body position, insufficient blood volume, or peripheral vasodilation caused by anesthetics. If necessary, the use of a PCA pump is paused, blood volume is replenished, and vasopressors are administered as needed [149].
Pruritus: Pruritus, induced by morphine-triggered histamine release, typically affects the head and neck, but may also spread to other body parts. Mild pruritus usually resolves within 1-2 days. For severe cases, patients should avoid scratching, and antihistamines such as diphenhydramine or promethazine can be administered. Nalmefene can be used for pruritus prevention [150].
Inhibition of bowel movements: Opioid analgesics used in PCA pumps, such as morphine and fentanyl, commonly inhibit bowel movements. Nurses should monitor patients’ bowel sounds, gas passage, and bowel movements. In abdominal surgery patients, symptoms such as abdominal distension, delayed gas passage, postoperative nausea, or vomiting may occur [151]. If no gas passage occurs within 3 days postoperatively, patients should be encouraged to increase activity, such as turning over or engaging in bedside mobility, and abdominal hot compresses may help stimulate bowel movements. Severe abdominal distension may require continuous gastrointestinal decompression or rectal gas evacuation as prescribed. Patients capable of eating should be encouraged to consume vegetables and fruits, like bananas, to aid bowel movements.
Chronic postsurgical pain (CPSP): Ineffectively controlled acute postoperative pain may lead to CPSP, particularly in patients with acute critical illness or those infected with COVID-19 prior to surgery. Studies have shown an increasing trend in CPSP incidence among postoperative patients with a history of COVID-19 infection and long COVID syndrome [152].
Psychological care: Optimal analgesic effects from PCA can only be achieved when combined with psychological care. Medical staff should provide reassurance and encouragement to boost the patient’s confidence in overcoming pain. Patients should be taught relaxation techniques, distraction strategies, and emotional adjustment, employing a combination of traditional Chinese and Western medicine approaches to proactively control pain [153].
Recommendations for the management of adverse reactions
During PCA administration, close monitoring is required, along with psychological care to help patients relax. Acupuncture and traditional Chinese medicine should be used as complementary treatments when necessary [154]. Recommendations for the management of adverse reactions (evidence level: B; recommendation grade: Strong; consensus rate: 100%).
Scope of the Expert Consensus
This Expert Consensus on the Standardized Clinical Application of Patient-Controlled Analgesia applies to adults and s and not to children, as the management and service of acute pain in younger children have unique characteristics [155].
Outlook
The development of PCA for pain management exemplifies the rapid advancement of clinical medicine in China. From its initial introduction to independent innovation, Ai-PCA has significantly enhanced the precision, reliability, and safety of pain management practices [156]. As AI continues to progress at an unprecedented rate, with technologies like Sora emerging following the release of ChatGPT 4.0 in 2024, PCA technologies must evolve to keep pace with these advancements, fostering further innovation and refinement in intelligent systems. The future of intelligent healthcare promises more standardized, safer, more effective, and higher-quality pain management services. By aligning the development of PCA with intelligent healthcare, we can ensure high-quality, standardized pain management [136,157,158]. This alignment will propel the fields of anesthesiology and pain medicine towards a future characterized by standardized, high-quality care driven by intelligent healthcare solutions [159,160].
Disclosure
The full version of this consensus was originally published in Chinese in Chinese Journal of Pain Medicine (Aug 2024, 20:484-508). This is the summary of the full report in English. There is no copyright issue for this consensus summary in English.
Conflict of Interest Statement
None.
References
- Raja SN, Carr DB, Cohen M, et al. The revised international association for the study of pain definition of pain: Concepts, challenges and compromises. Chin J Pain, 2020, 16(5):341-348.
- Tian X, Tao YX. Research progress in persistent postoperative pain. Chin J Anaesth, 2019, 39(6):655-659.
- She SZ, et al. Observation of the clinical effect of different combinations of buprenorphine in epidural patient self-controlled analgesia (PCEA). Chin J Pain, 1996, 3(4): 203-207.
- Liu GK, Huang YG, Luo AL, et al. Patient-controlled intravenous morphine and ketamine for postoperative analgesia. Chin J Anaesth, 2003, 23(6):416-418.
- Ruan XC, She SZ, et al. Standardization of acute pain treatment system. Chin J Pain, 2006, 12(2):6-8.
- Chinese Society of Anesthesiology Task Force on Management of Artificial Intelligent Patient-Controlled Analgesia. Expert consensus on management of artificial intelligent patient-controlled analgesia. Chin J Anaesth, 2018, 38(10):1161-1165.
- Huang WQ, Huang YG. Clinical study of accelerated intelligent postoperative patient self-controlled analgesia and labour analgesia. Guangdong Med J, 2020, 41(11):1081-1084.
- Cao HZ, Liu M, She SZ. Innovation of intelligent patient-controlled analgesia system and its compliance with regulations and standards. Guangdong Med J, 2020, 41(11):1088-1091.
- She SZ, Huang YG. Development and prospective strategy of patient-controlled analgesia in China. Transl Perioper Pain Med, 2018, 5(4):92-97.
- LI X, Xu JM, Liu ML, et al. New concept of perioperative analgesia: 3W analgesia. Chin J Anaesth. 2023, 43(11):1281-1286.
- Sechzer PH. Study in pain with analgesic-demand system. Anesth Analg, 1971, 50(1):1-10.
- Nijland L, Schmidt P, Frosch M, et al. Subcutaneous or intravenous opioid administration by patient-controlled analgesia in cancer pain: a systematic literature review. Support Care Cancer, 2019, 27(1):33-42.
- Mann C, Ouro-Bang'na F, Eledjam JJ. Patient-controlled analgesia. Curr Drug Targets, 2005, 6(7):815-9.
- Bridges KH, McSwain JR, Wilson PR. To infinity and beyond: The past, present, and future of tele-anesthesia. Anesth Analg, 2020, 130(2):276-284.
- McNicol ED, Ferguson MC, Hudcova J. Patient controlled opioid analgesia versus non-patient controlled opioid analgesia for postoperative pain. Cochrane Database Syst Rev, 2015, 2015(6):CD003348.
- Ahuja V, Thapa D, Ghai B. Strategies for prevention of lower limb post-amputation pain: A clinical narrative review. J Anaesthesiol Clin Pharmacol, 2018, 34(4):439-449.
- Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: A clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. Executive Committee, and Administrative Council. J Pain, 2016, 17(2): 131-57.
- Leong WL, Sultana R, Han NR, et al. Evaluation of vital signs-controlled, patient-assisted intravenous analgesia (VPIA) using remifentanil for labour pain. J Clin Anesth, 2021, 75: 110480.
- Kim MK, Nam SB, Cho MJ,et al. Epidural naloxone reduces postoperative nausea and vomiting in patients receiving epidural sufentanil for postoperative analgesia.Br J Anaesth, 2007, 99(2):270-5.
- Bower GH. Mood and memory. Am Psychol, 1981, 36(2): 129-48.
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ, 2008, 336(7650): 924-6.
- Refractory Cancer Pain Group of the Cancer Rehabilitation and Palliative Care Committee of the Chinese Anti-Cancer Association, Cancer Pain Group of the Pain Branch of the Chinese Medical Association. Expert consensus on cancer eruption pain (2019 edition). Chin Clin Oncol 2019, 46(6): 267-271.
- Wilson MJA, MacArthur C, Hewitt CA, et al. Intravenous remifentanil patient-controlled analgesia versus intramuscular pethidine for pain relief in labor (RESPITE): An open-label, multicenter, randomized controlled trial. Lancet. 2018, 392(10148):662-672.
- Yu AL, Critchley LA, Lee A, et al. Alfentanil dosage when inserting the classic laryngeal mask airway. Anesthesiology,2006,105(4): 684-8.
- Nair J, Rajan S, Paul J, et al. Efficacy and safety of intrathecal pentazocine as a sole anesthetic agent for lower limb surgeries. Anesth Essays Res, 2013, 7(1):49-53.
- Schnabel A, Reichel SU, Zahn PK, et al. Efficacy and safety of buprenorphine in peripheral nerve blocks: A meta-analysis of randomized controlled trials. Eur J Anaesthesiol, 2017, 34(9): 576-586.
- Gudin J, Fudin J. A narrative pharmacological review of buprenorphine: a unique opioid for the treatment of chronic Pain Ther, 2020, 9(1):41-54.
- Buer JK. Origins and impact of the term 'NSAID’. Inflammopharmacology, 2014, 22 (5): 263-7.
- Warden SJ. Prophylactic use of NSAIDs by athletes: A risk/benefit Phys Sportsmed, 2010, 38(1):132-8.
- Christopher K, Cheung JO, Adeola SS, et al. Postoperative pain management in enhanced recovery pathways. J Pain Res, 2022, 13:15:123-135.
- Fettes PD, Moore CS, Whiteside JB, et al. Intermittent vs continuous administration of epidural ropivacaine with fentanyl for analgesia during labour. Br J Anaesth, 2006, 97(3):359-64.
- Smet I, Vlaminck E, Vercauteren M. Randomized controlled trial of patient-controlled epidural analgesia after orthopaedic surgery with sufentanil and ropivacaine 0.165% or levobupivacaine 0.125%. Br J Anaesth, 2008, 100(1):99-103.
- Ngan Kee WD, Lee A. Multi-dimensional response-probability-dose curves for bupivacaine and ropivacaine epidural labour analgesia. Anesthesia, 2013, 68(4):368-76.
- Polley LS, Columb MO, Naughton NN, et al. Relative analgesic potencies of levobupivacaine and ropivacaine for epidural analgesia in Anesthesiology, 2003, 99(6):1354-8.
- Foster RH, Markham A. Levobupivacaine: a review of its pharmacology and use as a local anaesthetic. Drugs, 2000, 59(3):551-79.
- Bayazit EG, Karaaslan K, Ozturan K, et al. Effect of epidural levobupivacaine and levobupivacaine with fentanyl on stress response and postoperative analgesia after total knee replacement. Int J Clin Pharmacol Ther, 2013, 51(8): 652-9.
- Ocay DD, Li MMJ, Ingelmo P, et al. Predicting acute postoperative pain trajectories and long-term outcomes of adolescents after spinal fusion surgery. Pain Res Manag, 2020, 24:2020:9874739.
- Maher DP, Chen L, Mao J. Intravenous ketamine infusions for neuropathic pain management: a promising therapy in need of optimisation. Anesth Analg, 2017, 124(2):661-674.
- Pendi A, Field R, Farhan SD, et al. Perioperative ketamine for analgesia in spine surgery: a meta-analysis of randomized controlled trials. Spine, 2018, 43(5):E299-E307.
- Wang L, Johnston B, Kaushal A, et al. Ketamine added to morphine or hydromorphone patient-controlled analgesia for acute postoperative pain in adults: A systematic review and meta-analysis of randomized Can J Anaesth, 2016, 63(3):311-25.
- Chazan S, Buda I, Nesher N, et al. Low-dose ketamine via intravenous patient-controlled analgesia device after various transthoracic procedures Low-dose ketamine via intravenous patient-controlled analgesia device after various transthoracic procedures improves analgesia and patient and family satisfaction. Pain Manag Nurs, 2010, 11(3):169-76.
- De Oliveira GS Jr, Castro-Alves LJ, Khan JH, et al. Perioperative systemic magnesium to minimize postoperative pain: a meta-analysis of randomized controlled Anesthesiology, 2013, 119(1):178-90.
- Huang YG, She SZ, Huang WQ, et al. Intelligent patient-controlled analgesia [M]. Beijing: People's Health Publishing House, 2023, 10-76.
- Carollo A, Tobar A, Hernandez C.A rule-based postoperative pain controller: simulation results. Int J Biomed Comput, 1993, 33(3-4):267-76.
- Rudolph H, Cade JF, Morley PT, et al. Smart technology improves patient- controlled analgesia: a preliminary report. Anesth Analg, 1999, 89(5):1226-32.
- Hull CJ, Sibbald A. Control of postoperative pain by interactive demand analgesia. Br J Anaesth 1981, 53(4):385-91.
- Wang J,She SZ. Morphine pharmacokinetics and blood concentration monitoring in patient-controlled analgesia. Chin. J. Clin. Pharm,1999,8(5): 282-284.
- Grass JA. Patient-controlled analgesia. Anesth Analg, 2005, 101(5S):S44-S61.
- Wei SM, Sun XD. Clinical effect of hydromorphone and oxycodone in patient-controlled intravenous analgesia after thoraco-scopic radical resection of lung cancer. Journal of Practical Medicine, 2021, 37(24): 2908-2913.
- Jacobs OLR, Bullingham RES, Lammer P, et al. Modelling estimation and control in the relief of postoperative pain. Automatica, 1985, 21(4):349-360.
- She SZ, Xu XB. Controversy over the effectiveness of preemptive analgesia and the current status of research on prophylactic analgesia. Journal of Practical Pain, 2007, 3(6): 270-275.
- Zhang QF, Zhang R, He M, et al. A survey of perioperative pain treatment and management in China.Chin J Anaesth, 2017, 37 (12):1409-1413.
- Bos EME, Hollmann MW, Lirk P. Safety and efficacy of epidural analgesia. Curr Opin Anaesthesiol, 2017, 30(6):736-742.
- Hong X, Huang YG, Luo AL. Standardized management of postoperative analgesia. Chin J Anaesth, 2005, 25(10):798-799.
- Ilfeld BM. Continuous peripheral nerve blocks: an update of the published evidence and comparison with novel, alternative analgesic modalities. Anesth Analg, 2017, 124(1):308-335.
- Pöpping DM, Elia N, Marret E, et al. Protective effects of epidural analgesia on pulmonary complications after abdominal and thoracic surgery: A meta-analysis. Arch Surg, 2008, 143(10):990-9.
- She SZ, Liu JY, Liu R, et al. Subarachnoid - Clinical study of PCEA after combined epidural Chin J Anaesth, 1998, 18(6):378-379.
- He L, She SZ, Xie XQ. Efficacy of patient-controlled epidural analgesia with different concentrations of fentanyl added to levobupivacaine after abdominal total hysterectomy. Chin J Anaesth, 2007, 27(2):173-176.
- Xu XB, She SZ, Xu LX, et al. Comparison of analgesic effect of PCEA with multi-model analgesic for interventional uterine artery embolization. International Journal of Anesthesiology and Resuscitation, 2006, 27(4):221-223.
- LI RS, She SZ, Zhou HF, et al. A comparison of efficacy of continuous epidural infusion of 0.2% levobupivacaine and 0.2% ropivacaine for postoperative pain relief. Chin J of Anaesth, 2004, 24(1):73-75.
- Zhou HF, She SZ, Xu LX, et al. Effects of continuous epidural infusion of ropivacaine combined with clonoxicam PCA and changes of plasma IL6 and IL10 in patients. Chin J Pain, 2006, 12(1):72-75.
- Deng WJ, Qiu CY, Hou J, et al. Application of flexible reinforced wire catheter in epidural gap placement during anesthesia operation. Guangdong Med J, 2017, 38(17):2665-2669.
- Patel AB, Kerins GJ, Sites BD, et al. Differences in the association between epidural analgesia and length of stay by surgery type: an observational study by surgery type: an observational study. Reg Anesth Pain Med, 2024, Jan 29: rapm-2023-105194.
- She SZ, Liu JY, Xu LX, et al. Clinical effects of different combinations of buprenorphine in epidural patients with self-controlled analgesia (PCEA).Chin J Pain, 1996, 2(4):203-207.
- Patel M. Combined spinal and extradural anesthesia. Anesth Analg, 1992, 75(4): 640-1.
- Chapman CR. Psychological aspects of postoperative pain control. Acta Anesthesiol Belgica, 1992, 43(1):41-45.
- Wang R, Wang SS, Dang N, et al. From patient-controlled analgesia to artificial intelligence-assisted patient-controlled analgesia: practices and Frontiers Med, 2020, May 22:7:145.
- Scott DA, Charnley DM, Mooney PH, et al. Epidural ropivacaine infusion for postoperative analgesia after major lower abdominal surgery--a dose finding study. Anesth Analg, 1995, 81(5):982-6.
- Lakra A, Grosso M, Jennings EL, et al. Improved pain control with adductor canal block using liposomal bupivacaine after total knee replacement: a retrospective cohort study. Arthroplast Today, 2019, 5(3): 325-328.
- Taylor SA. Safety and Satisfaction Provided by Patient-Controlled Analgesia. Dimensions of Critical Care Nursing Dccn 2010, 29(4):163-6.
- Baek W, Jang Y, Park CG, et al. Factors influencing satisfaction with patient-controlled analgesia among postoperative patients using a generalized ordinal logistic regression model. Asian Nurs Res, 2020, 14(2):73-81.
- Davis KD, Flor H, Greely HT, et al. Brain imaging tests for chronic pain: medical, legal and ethical issues and recommendations. Nat Rev Neurol, 2017, 13(10):624-638.
- Man Y. Design and application of wireless analgesic pump system based on Zigbee technology. Medical Equipment, 2012, 25(12):16-17.
- Zhao X, Li XH, Liu XH. Principle and application of wireless analgesic monitoring system. China Digital Medicine, 2014, 9(3):15-17.
- Yang SY, Yu JJ, Zhu SH. Application of wireless analgesia information system in anesthesiology department. China Medical Equipment, 2014, 11(1):57-59.
- Yang GYY, Zuo SS, Wang PF, et al. Virtual pain unit is associated with improvement of postoperative analgesia quality: A Retrospective Single-Center Clinical Study. Pain Ther, 2023, 12(4):1005-1015.
- Tawfic QA, Faris AS. Acute pain service: past, present and future. Pain manag, 2015, 5(1):47-58.
- Chemali ME, Eslick GD. A meta-analysis: Postoperative pain management in colorectal surgical patients and the effects on length of stay in an enhanced recovery after surgery (ERAS) setting. Clin J Pain, 2017, 33(1):87-92.
- Cao HZ, Men YH, Tu WF. Intelligent technology is an efficient means to improve analgesia safety and quality control. Anaesthesia Safety and Quality Control, 2017, 1(3):111-116.
- Wang D, Guo YH, Yin Q, et al. Analgesia quality index improves the quality of postoperative pain management: a retrospective observational study of 14,747 patients between 2014 and 2021. BMC Anesthesiol, 2023, 23(1):281.
- Arendt KW, Lindley KJ. Obstetric anesthesia management of the patient with cardiac disease. Int J Obstet Anesth,2019,Feb:37:73-85.
- Tawfic QA, Bellingham G. Postoperative pain management in patients with chronic kidney disease. J Anaesthesiol Clin Pharmacol, 2015, 31(1):6-13.
- Wang R, Wang SS, Duan N, Wang Q. From patient-controlled analgesia to artificial intelligence-assisted patient-controlled analgesia: Practices and perspectives. Front Med, 2020, May 22:7:145.
- Manion SC, Brennan TJ. Thoracic epidural analgesia and acute pain management. Anesthesiology, 2011, 115(1):181-8.
- Liu JY, She SZ, Suo K, et al. Pharmacokinetics of morphine during intravenous PCA. Chin J Anaesth, 2001, 21(4):251-252.
- She SZ, Suo K, Liu JY, et al. Study on the clinical effects and blood concentration of different PCA doses of epidural morphine. Chin J Anaesth.1999, 19(6):376-377.
- Li QB, Shu HH, Ye F, et al. Different doses of pentazocine inhibit the analgesic effect of morphine. Chin J Pain, 2016, 22(2):102-108.
- Cai Z, Zeng XL, Gu XY, et al. Efficacy of hydromorphone as a substitute for morphine to improve postoperative analgesia. Journal of Sun Yat-sen University(Medical Science Edition), 2016, 37(4):579-584.
- Gong ZY, Ye TH, Zhu B, et al. Comparison of patient-controlled analgesia with buprenorphine versus morphine after abdominal hysterectomy. Chin J Anaesth, 2003, 23(4): 272-274.
- Sun ZT, Zhu ZF, Zhu L, et al. Effect of sufentanil combined with nalbuphine on postoperative patient-controlled analgesia after gynecological laparoscopic surgery. Tianjin Med J, 2019, 47(1):55-58.
- Wang WJ, Ren LY, Gong YH, et al. Efficacy of oxycodone hydrochloride for patient-controlled intravenous analgesia after general surgery. Journal of Chinese Academy of Medical Sciences, 2020, 42(1):91-95.
- Xu X, Wu XM, Xue ZZ,et al. Efficacy and safety of oxycodone hydrochloride injection for postoperative analgesia in patients undergoing operation under general anesthesia: A prospective, randomized, blinded, multicenter, positive-controlled, clinical trial. Chin J Anaesth, 2013, 33(3):269-274.
- Zhang Q, Zhang XT, Zhang R, et al. Analgesic effect and safety flurbiprofen axel injection combined with lidocaine hydrochloride injection for patient lumbar spine surgery. Chinese Journal of Clinical Pharmacology, 2022, 38(1):6-9.
- Luo XJ, Zhong XL, Zheng B, et al. Effects of different doses of dexmedetomidine on the analgesic effect of sufentanil intelligent patient-controlled intravenous analgesia after abdominal surgery. Guangdong Med J, 2020, 41(11):1128-1133.
- Niu YF, Zhu Y, Chang J. Study on the application value of sufentanil and sufentanil combined with dexmedetomidine in postoperative analgesia of lung cancer. Chinese Drugs and Clinics, 2021, 21(23):3903-3905.
- Schug SA, Palmer GM, Scott DA, et al. Acute pain management: scientific evidence, 2015. Med J Aust, 2016, 204(8):315-7.
- She SZ, Suo K, Liu JY, et al. Clinical study on the pharmacodynamics and blood concentration changes of intravenous and epidural morphine PCA.JCA, 1999, 15(5):263-265.
- Liu JY, She SZ, MOK MS. The effects of postoperative patient-controlled epidural analgesia with different combinations of tramadol and/or buprenorphine: a comparative study. Chin J Anaesth, 1998, 18(5):313-315.
- Han TF, Zhang J, Luo JR, et al. Observation on the effect of different doses of hydromorphone in patient-controlled epidural analgesia after cesarean section. Clinical Medical Engineering, 2020, 27(12):1713-1714.
- Wang XL, She SZ, Xie XQ. Effects of different mode of extradural PCA with Pentazocine on the post-operative analgesia of cesarean section. Guangdong Med J, 2008, 29(11):1906-1909.
- Guo XY, Huang YG, Jin YF, et al. Patient-controlled analgesia: A comparison of intravenous versus subcutaneous morphine. JCA, 1999, 15(2):78.
- She SZ, Xu LX, Liu JY, et al. A randomized double-blind comparison of efficacy of patient controlled epidural analgesia with combinations of fentanyl and other drugs for postoperative pain. Chin J Anaesth, 1997, 17(4):245-247.
- Nguyen E, Lim G, Ross SE. Evaluation of therapies for peripheral and neuraxial opioid-induced pruritus based on molecular and cellular discoveries. Anesthesiology. 2021, 135(2):350-365.
- Hu XL, Dai QQ, Liang YP, et al. Effect of oxycodone for patient-controlled intravenous analgesia after hip arthroplasty in elderly patients, Chin J Anaesth, 2016, 36(10):1229-1231.
- She SZ, Xu XB, Liu JY, et al. Comparison of postoperative analgesic effects between ropivacaine and bupivacaine in patients with patient-controlled epidural Guangdong Med J, 1999, 20(9):669-671.
- Boudreauh D, Brasseul L Samii K, et al. Comparison of continuous epidural bupivacaine infusion plus either continuous epidural infusion or patient-controlled epidural injection of fentanyl for postoperative analgesia. Anaesth Analg, 1991, 73(2):132-7.
- Glass PS, Estok P, Ginsberg B, et al. Use of patient-controlled analgesia to compare the efficacy of epidural to intravenous fentanyl administration. Anaesth Analg, 1992, 74(3):345-51.
- Motamed C, Farhat F, Rémérand F, et al. An analysis of postoperative epidural analgesia failure by computed tomography epidurography. Anesth Analg, 2006, 103(4):1026-32.
- Clemente A, Carli F. The physiological effects of thoracic epidural anesthesia and analgesia on the cardiovascular, respiratory and gastrointestinal systems. Minerva Anestesiol, 2008, 74(10):549-63.
- Egches RC, writer WDR, Ansley D, et al. Continous epidural ropivacaine 0.2% for analgesia after lower abdominal surgery. Anaesth Analg, 1997, 84(4):784-90.
- Liu SS, Morre JM, Luo AM, et al. Comparison of three solutions of ropivacaine/fentanyl for postoperative patient-controlled epidural analgesia. Anaesthesiology, 1999, 90(3):727-33.
- Schug SA, Scott DA, Payne J, et al. Postoperative analgesia by continuous extradural infusion of ropivacaine after upper abdominal surgey. Br J Anaesth, 1996, 76(4):487-91.
- She SZ, Yan Y, Xu XB, et al. Comparison of the effects of PCA effects with and without morphine loading dose under background infusion of ropivacaine. Chin J Anaesth, 2002, 18(1):35-39.
- She SZ, Xu XB, Xiao JB, et al. Effects of continuous epidural infusion of at different rates of ropivacaine on morphine PCA consumption. Chin J Anaesth, 2000, 20(9):570-571.
- Heil JW, Nakanote KA, Madison SJ, et al. Continuous transversus abdominis plane (tap) blocks for postoperative pain control after hernia surgery: a randomized, triple-masked, placebo-controlled study. Pain Med, 2014, 15(11):1957-64.
- Zhang J, Zhao MY, Pei LJ, et al. Epidural labor Analgesia: comfort and safety run in parallel, with teaching and quality control complementing each other. Journal of Concordant Medicine, 2024, 15(2):246-250.
- Peng LH, Ren L, Qin PP, et al. Continuous femoral nerve block versus intravenous patient-controlled analgesia for knee mobility and long-term pain in patients receiving total knee replacement: a randomized controlled trial. Evid Based Complement Alternat Med, 2014: 2014:569107.
- Bao CY, Zhao Y, He H. Effects of sufentanil combined with different doses of ropivacaine epidural labour analgesia on maternal pregnancy outcome. Shanxi Med J, 2022, 51(1):53-56.
- Jiang HW, Xu SY, Fang MJ. Ropivacaine combined with sufentanil or fentanyl for labor analgesia in latent phase. JCA, 2015, 31(3):221-223.
- Yu YB, Lin R, Xu ZD. Concurrent control study on the effect of remifentanil parturient-controlled intravenous analgesia and sufentanil combined ropivacaine parturient-controlled epidural analgesia in labor. Chinese Journal of Maternal and Child Clinical Medicine (Electronic Edition), 2016, 12(3):326-333.
- Ren Jie, Wei Xiaoyong, Yang Bo, et al. Comparison of the effects of patient-controlled intravenous labor analgesia with remifentanil and epidural labor analgesia: a meta-analysis. Chin J Anaesth, 2020, 40(9):1121-1124.
- Wang Xiuyan. PCEA epidural block for intractable angina pectoris. Zhongyuan Medical Journal, 2002, 29(4): 40-41.
- LI JH, Chen KL, Xia ZY, et al. High-level epidural block for intractable angina pectoris. JCA, 2000, 16(8): 412.
- Loftus RW, Yeager MP, Clark JA, et al. Intraoperative ketamine reduces perioperative opiate consumption in Opiate-dependent patients with chronic back pain undergoing back Anesthesiology, 2010, 113(3):639-46.
- Kurd MF, Kreitz T, Schroeder G, et al. The role of multimodal analgesia in spine surgery. J Am Acad Orthop Surg, 2017, 25(4):260-268.
- Li J, Ajiboye RM, Orden MH, et al. The effect of ketorolac on thoracolumbar posterolateral fusion: A systematic review and meat-analysis. Clin Spine Surg, 2018, 31(2):65-72.
- Klatt JW, Mickelson J, Hung M, et al. A randomized prospective evaluation of 3 techniques of postoperative pain management after posterior spinal instrumentation and fusion. Spine, 2013, 38(19):1626-31.
- Sheets NW, Davis JW, Dirks RC, et al. Intercostal nerve block with liposomal bupivacaine vs epidural analgesia for the treatment of traumatic rib fracture. J Am Coll Surg, 2020, 231(1):150-154.
- Liu XL, Wan CF, Ma K, et al. Chinese expert consensus on subcutaneous continuous infusion for cancer pain treatment (2020 edition). Chin J Pain, 2020, 16(2):85-91.
- Taketa Y, Irisawa Y, Fujitani T. Comparison of ultrasound-guided erector spinae plane block and thoracic paravertebral block for postoperative analgesia after video-assisted thoracic surgery: A randomized controlled non-inferiority clinical trial. Reg Anesth Pain Med, 2019, Nov 8:rapm-2019-100827.
- Hara K, Sakura S, Yokokawa N, et al. Incidence and effects of unintentional intraneural injection during ultrasound-guided subgluteal sciatic nerve block. Reg Anesth Pain Med, 2012, 37(3):289-93.
- Subgroup of Critical Respiratory Diseases, China Clinical Practice Guideline Alliance. Expert consensus on analgesic management in severe adult patients in China[J]. Chinese Journal of Critical Care & Intensive Care Medicine (Electronic Edition), 2023, 9(2):97-115.
- Van Boekel RL, et al. Acute pain services and postsurgical pain management in the Netherlands: A survey. Pain Pract, 2015, 15(5):447-54.
- Wan CF, Meng QZ, Wang YW, et al. Patient-controlled subcutaneous analgesia using sufentainil or morphine in home care treatment in patients with stage ?-? cancer: A multi-centre randomized controlled clinical trial. Cancer Med, 2020, 9(15):5345-5352.
- Kondasinghe JS, Tuffin PHR, Findlay FJ. Subcutaneous patient-controlled analgesia in palliative care. J Pain Palliat Care Pharmacother, 2021, 35(3):163-166.
- Zhuang YW, Wang Y, He B, et al. Molecular recognition of morphine and fentanyl by the human μ-opioid receptor. Cell, 2022, 185(23):4361-4375.
- Peyton PJ, Wu CY. Nitrous oxide-related postoperative nausea and vomiting depends on duration of exposure.Anesthesiology, 2014, 120(5):1137-45.
- Lee J, Faraoni D, Lee S, et al. Incidence and risk factors for postoperative vomiting following atrial septal defect repair in children. Pediatric Anaesth, 2016, 26(6):644-8.
- Beattie WS, Badner NH, Choi P. Epidural analgesia reduces postoperative myocardial infarction: A meta-analysis. Anesth Analg, 2001, 93(4):853-8.
- Mouraux A, Iannetti GD. The search for pain biomarkers in the human brain. Brain, 2018, 141(12):3290-3307.
- Gan TJ, Kranke P, Minkowitz HS, et al. Intravenous amisulpride for the prevention of postoperative nausea and vomiting: two concurrent, randomized, double-blind, placebo-controlled trials. Anesthesiology, 2017, 126(2):268-275.
- Bingham AE, Fu R, Horn JL, et al. Continuous peripheral nerve block compared with single-injection peripheral nerve block: a systematic review and meta- analysis of randomized controlled trials. Reg Anesth Pain Med, 2012, 37(6):583-94.
- Singh PM, Borle A, Rewari V, et al. Aprepitant for postoperative nausea and vomiting: a systematic review and meta-analysis. Postgrad Med J, 2016, 92(1084):87-98.
- Long QE, Zhao L, Zhang CL. Retrospective analysis of 972 cases of adverse drug reactions/event opioids. J Pharmacoepidemiol, 2019, 28(5):314-318.
- Wang SS, Duan N, Li XG, et al. Effects of artificial intelligent patient-controlled analgesia on the adverse reactions and satisfaction for postoperative patients. Guangdong Med J, 2020, 41(11):1097-1100.
- Zhao XB. Application of wireless analgesia system in maternal analgesia care after cesarean section. Zhong guan cang Medicine, 2019, 31(12):171-172.
- Xie H, Ma ZL, Qiu YD, et al. Working mode and effect of clinical pharmacists involved in postoperative patient-controlled analgesia pump management. Chin Pharm J, 2019, 54(19):1622-1625.
- Gu JF, Jiang WX, Zhou DD, et al. Exploring the way and effect of implementing personalised psychological care for cancer pain patients. Psychological Monthly, 2019, 14(9):34-35.
- Zhong XM, Su YQ, Ye XQ. Nursing intervention of non-invasive analgesic delivery in clinical application of obstetrics. China Contemporary Medicine, 2016, 23(34):89-91.
- Raja SN, Carr DB, Cohen M, et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain, 2020, 161(9):1976-1982.
- Wu JL, Tian Y, Nie WH, et al. Risk factors for postoperative nausea and vomiting in surgical patients undergoing patient-controlled intravenous analgesia. Journal of Concordant Medicine, 2024, 15(2):366-374.
- Che L, Yu JW, Jin D, et al. Analysis of the incidence of chronic postoperative pain and its risk factors in patients with preoperative novel coronavirus infection: A two-way cohort study. Journal of Concordant Medicine, 2024, 15(2):344-350.
- Yan H, Chen JJ, Luo JW, et al. Prophylactic use of IV nalmefene to prevent epidural opioid-induced pruritus: A multicenter, randomized clinical trial. J Clin Anesth, 2024, Feb:92:111301.
- Xue JJ, Xu ZQ, Wang Q, et al. Clinical practice guidelines for prevention and treatment of postoperative gastrointestinal disorder with Integrated Traditional Chinese and Western Medicine(2023). J Evid Based Med. 2024, 17(1):207-223.
- Burjek NE, Hafeman M, Guthrie D, et al. Perioperative use of gabapentinoids in pediatric patients. Anesthesiol Perioper Sci. 2023; 1(3):21-24.
- Xu XB, She SZ. Refined intelligence leads clinical pain treatment into a new journey. Guangdong Medicine, 2022, 43(10):1189-1195.
- Deng XM, Yao SL, Yu BW, et al. Modern Anesthesiology [M]. 5th ed. Beijing People's Health Press, 2020, 2784-2818.
- She SZ. Efforts to promote the rapid development of intelligent patient-controlled analgesia technology. Chin J Pain, 2023, 19(1):3-5.
- She SZ, Yao SL, Yu WF, et al. Patient-controlled analgesia should be standardized and developed with high quality towards digital intelligent medicine. Chin J Pain, 2024, 20(4):481-483.
- Xiong LZ. Innovation promotes high-quality development of anaesthesia discipline. Chin J Anaesth, 2024, 44(1):1-2.
Table of Contents
- Abstract
- Keywords
- Introduction
- Origin and Development of PCA
- Definition and Advantages of PCA
- Consensus Development Methods and Basis
- PCA Analgesic Medications
- Common Clinical Applications of PCA
- Pre-PCA Assessment and Patient-Family Education
- Structure of Artificial Intelligence PCA (Ai-PCA)
- PCA Standardized Management System
- Typical PCA Formulations
- Monitoring and Management of Adverse Reactions to PCA
- Scope of the Expert Consensus
- Outlook
- Disclosure
- Conflict of Interest Statement
- Figure 1
- Figure 2
- Table 1
- Table 2
- References