Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/192
Review Article | Volume 11 | Issue 3 Open Access
Advances in the Therapeutic Effects of Ketamine on Cancer Pain Related Depression
Jin-Feng Wang1#, Ming-Yue Geng2#, Huan Yu3, Qin Yin3,4* and Wei Cheng2,3,4*
1Xuzhou Central Hospital, Xuzhou, 221009, P.R. China
2The Affiliated Huai'an No.1 People's Hospital of Nanjing Medical University, Huai'an 223300, P.R. China
3Jiangsu Province Key Laboratory of Anesthesiology, Xuzhou Medical University, Xuzhou 221004, P.R. China
4The Affiliated Hospital of Xuzhou Medical University, Xuzhou 221002, PR. China
#indicates equal contribution
Wei Cheng, MD, The Affiliated Huai'an No.1 People's Hospital of Nanjing Medical University, Huai'an 223300, China; The Affiliated Hospital of Xuzhou Medical University, Xuzhou 221002, PR. China, E-mail: 53974314@qq.com;Qin Yin, MD, The Affiliated Hospital of Xuzhou Medical University, Xuzhou 221002, PR. China, E-mail: 810780794@qq.com
Editor: Renyu Liu, MD; PhD; Professor, Department of Anesthesiology and Critical Care, Perelman School of Medicine at the University of Pennsylvania, Center of Penn Global Health Scholar, 336 John Morgan building, 3620 Hamilton Walk, Philadelphia, PA 19104, USA, Fax: 2153495078, E-mail: RenYu.Liu@pennmedicine.upenn.edu
Received: December 11, 2020 | Accepted: December 14, 2024 | Published: December 16, 2024
Citation: Wang JF, Geng MY, Yu H, Yin Q, Cheng W. Advances in the Therapeutic Effects of Ketamine on Cancer Pain Related Depression; 11(3):653-671
Abstract
Pain is a prevalent and significant adverse effect experienced by individuals diagnosed with cancer. Cancer patients with anxiety and depression can serve to exacerbate their pain. Cancer pain-related depression (CPD) is defined as a depressive state caused by the presence of ongoing cancer-related pain. CPD represents a significant physical and mental health burden for patients.
In clinical settings, ketamine has been shown to be an effective medication for cancer pain and depression. Ketamine has been shown to produce significant improvements in late-stage cancer pain and depression. This article reviews the development of ketamine in the treatment of cancer pain and depression, presents case reports of ketamine-assisted opioid therapy for CPD, and discusses related reports of adverse effects and immunoregulatory effects of ketamine, as well as its impact on tumor recurrence.
Keywords
Ketamine, Cancer pain, Depression, Ketamine infusion, Pain therapy
Introduction
About 19.3 million new cancer cases and almost 10.0 million deaths from cancer occurred in 2020 worldwide [1]. The growth of cancer is attributed to the aging population and the development of cancer screening technologies. Psychological factors such as depression play an important role in the development and prognosis of tumors. In clinical oncology, it is essential to diagnose and treat depression in cancer patients at an early stage [2,3].
Cancer-related depression (CRD) refers to a pathological depressive state or syndrome along with diagnosing and treating malignant tumors, making it one of the most common adverse emotions among cancer patients [4]. After diagnosis, cancer patients face the integration of multiple treatment modalities and bear greater psychological distress and anxiety during the transition between treatment approaches [5]. The psychological state of anxiety and depression, coupled with a negative attitude toward treatment, can lead to cancer progression or recurrence, which negatively affects treatment outcomes, prognosis, and overall quality of life [4].
Review of Research on the Comorbidities of Depression and Cancer
Tumors can cause patients to become depressed and subsequently develop depression. Conversely, depression can lead to the onset, development, recurrence, and metastasis of tumors. The two are interrelated and inseparable [5].
Increased incidence of depression in people with cancer
Cancer can increase the likelihood of depression in two ways. First, the occurrence of depression is associated with the abnormal immune dysfunction and disruption of the neuroendocrine system during the onset of cancer, which is accompanied by strong psychological reactions caused by severe diagnostic results and deteriorating physical conditions [5,6]. Second, cancer pain and toxic side effects caused by long-term radiotherapy, chemotherapy and immunotherapy can also lead to the onset and even worsening of depression in cancer patients [7].
Depression may increase cancer incidence
Depression-induced dysfunction of the HPA axis affects the immune status of cancer patients through neuroendocrine immune regulation [8,9].
Second, patients with depression have higher levels of inflammatory factors than the general population. Over time, these inflammatory factors can stimulate the body and damage cellular DNA, leading to mutations and tumors [10,11]. The above evidence suggests that depression may affect tumor incidence and prognosis in cancer patients through neuroendocrine dysfunction and decreased immune function.
Third, research shows that breast cancer patients with depression are more prone to tumor recurrence [12]. Due to the interaction between depression and cancer, the treatment of CRD is crucial for the comprehensive management of malignant tumors.
Research on Cancer Pain and Depression
Comorbidity of cancer pain and depression
Hoos, et al. first reported that cancer patients suffer from pain-induced depression. The pain-induced depression will affect the effectiveness of analgesic treatment [13,14]. Kai hoi Sze, et al. found in a survey of 1721 cancer patients that 60% of them had pain symptoms, anxiety, depression, and a significant positive correlation with physical pain [15]. Zabora, et al. [16] found that CRD can shorten the survival time of cancer patients by 10% to 20%. Stommel, et al. [17] analyzed 871 cancer patients and found that patients with depression were more likely to die within 19 months compared to patients without depression. Therefore, it is believed that the prognosis of cancer patients can be assessed based on the degree of depression.
The above research suggests that depression is an adverse complication in cancer patients, and the reasons for its occurrence may include: (1) Cancer is a significant psychological stimulus for patients. When patients know the diagnosis of cancer, they immediately experience fear and sadness after initially denying it, and cannot accept the fact that they will give up their loved ones and leave this world forever; (2) Worry about the excessive economic burden caused by the disease; (3) Uncontrollable pain may also lead to anxiety and depression in patients; (4) When patients lack understanding of their own condition and hope for early diagnosis, they may also experience anxiety and depression; (5) Patients have more anxiety after learning that they have cancer, and the incidence of anxiety and depression is higher; (6) Some unbearable examinations and treatment procedures may also aggravate depression. Therefore, good pain relief and appropriate psychological or antidepressant treatment are of great importance for cancer patients [18].
A randomized controlled trial published in 2004 showed that pain levels might predict the onset of depression [19,20]. Depression is one of the most common mental illnesses caused by cancer and is often misdiagnosed [18]. Depression and pain typically co-occur in patients [21,2]. It is also reasonable to assume that the impact of cancer pain on quality of life and treatment outcomes is related to comorbid depression. Given our understanding of the common central pathways and neuropharmacology of pain, depression, and anxiety, it seems reasonable to conclude that they are closely related [18].
Cancer pain related depression
The new concept of cancer pain related depression: Cancer pain-related depression (CPD) refers to a depressive state caused by persistent cancer-related pain. CPD not only imposes a great physical burden on patients, but also seriously affects their mental health. Persistent pain not only makes patients physically uncomfortable, but can also cause or exacerbate psychological symptoms such as depression, significantly reducing their quality of life. CPD not only interferes with patients' daily life and social activities, but can also interfere with the effectiveness of cancer treatment.
The diagnosis of CPD requires a combination of symptom presentation, medical history, assessment tools, and clinical examination. It must also be differentiated from common depressive disorders, medication side effects, and other mental and physical illnesses. Effective cancer pain management and psychological intervention are important to alleviate depression and improve patients' quality of life. Through interdisciplinary collaboration, clinicians can better identify and treat cancer-related depression and improve patients' overall health status.
Preliminary recommendations for the diagnostic basis of CPD
1) Symptoms of depression: Patients experience symptoms of depression such as low mood, loss of interest, worthlessness, fatigue, difficulty concentrating, and sleep disturbance during cancer pain [23,24]. These symptoms persist for at least two weeks and significantly interfere with the patient's daily life and functioning.
2) Medical history: Understand the patient's cancer type, course, level of pain, and impact on daily life. Cancer and its treatments (such as chemotherapy and radiation) are often associated with severe pain, which can be an important trigger for depressive symptoms [25,26].
3) Assessment tools: (1) Depression-related assessment tools include the use of standardized depression assessment scales, such as the Beck Depression Inventory (BDI), Hamilton Depression Scale (HAMD), etc., to objectively evaluate the degree of depression in patients. These scales can help clinicians quantify patients' depressive symptoms as a diagnostic reference [23,24]: Numerical Rating Scale (NRS), Visual Analog Scale (VAS), Faces Pain Scale (FPS), Brief Pain Inventory (BPI), McGill Pain Questionnaire (MPQ), and Pain Relief Scale (PRS) [25,26]. Common assessment scales for tumor patients include: ECOG Performance Status (Eastern Cooperative Oncology Group), Karnofsky Performance Status (KPS), FACT-G (Functional Assessment of Cancer Therapy-General), QLQ-C30 (Quality of Life Questionnaire Core 30), MDASI (MD Anderson Symptom Inventory), Brief Pain Inventory (BPI), NCCN Distress Thermometer [25,26].
4) Clinical examination: Neuroimaging (such as MRI, CT, etc.) and laboratory tests may be necessary to rule out other physical diseases that may cause depressive symptoms. In particular, it is necessary to exclude depressive symptoms caused by brain metastases, metabolic abnormalities, etc. [25,26].
5) Expert consensus and guidelines: Diagnose depression based on international and national diagnostic guidelines [23,24], combined with the special circumstances of cancer patients [25,26].
Differential Diagnosis of CPD
The purpose of differential diagnosis is to distinguish CPD from other possible causes of depressive symptoms, including the following situations:
1) General depressive disorder: The core features of general depressive disorder are symptoms of low mood, loss of interest, and worthlessness that may be accompanied by physical pain, but the pain is not the primary cause of the symptoms [23,24]. The depressive symptoms of cancer-related depression are often accompanied by persistent cancer pain, and pain is an important trigger for their depressive symptoms [25,26].
2) Drug side effects: Some cancer treatment drugs (such as steroids [27], interferons [28], etc.) may cause depressive symptoms. A detailed medication history and the temporal relationship between symptoms can help distinguish between drug-induced depressive symptoms and cancer-related depression.
3) Other mental illnesses (anxiety disorders [29], post-traumatic stress disorder (PTSD) [30], etc), may present with similar depressive symptoms. A thorough psychiatric evaluation can help distinguish these conditions from cancer-related depression.
4) Physical illnesses such as hypothyroidism [31], brain tumors [32], infections [33], etc. may cause symptoms similar to depression. Clinical and laboratory tests can rule out depressive symptoms caused by these physical illnesses.
In conclusion, cancer pain may lead to or aggravate depression, and depressive states make cancer pain difficult to control and may promote tumor progression. Therefore, active and effective control of cancer pain can help improve patients' quality of life, treatment compliance, and even, to some extent, patient prognosis. Therefore, effectively treating CPD can help improve cancer treatment efficacy, treatment compliance, and even, to some extent, patient prognosis.
Research has confirmed that pain and depression share many common regulatory mechanisms, and exploring the optimal treatment approach and plan for CPD is an urgent issue that clinicians need to address.
Research on Ketamine Reducing Cancer Pain and Depression
Basic introduction to the pharmacological mechanism of ketamine
Ketamine is an N-methyl-D-aspartate (NMDA) receptor antagonist: Low doses ketamine can produce analgesic effects, while high doses can produce anesthetic and potent analgesic effects. As an anesthetic, ketamine also have some adverse effects including pseudopsychotic symptoms, gastrointestinal reactions visual impairment, and dizziness [34].
NMDA receptors consist of two subunits, GluN2A and GluN2B. S-ketamine acts primarily on the GluN2A subunit to induce hypnosis and primarily on the GluN2B subunit as part of the analgesic process [35].
Ketamine can non-competitively antagonize NMDARs. It reduces the activity of calcium channels and Ca 2+ influx which regulate pain sensitivity [35]. With repeated stimulation of spinal C-fibers, NMDA receptors increase spontaneous fiber activity and receptive fields [36-38].
Effects of ketamine on other receptors: S-ketamine can also activate opioid receptors. S-ketamine also blocks sodium channels, inhibits NO release, suppresses muscarinic acetylcholine receptors, stimulates the sympathetic nervous system, induces NA release, promotes endogenous opioid release, and activates opioid receptors. After binding to the receptor, it produces further analgesic effects [39,40].
In the experimental model, ketamine dose-dependently inhibits the expression of monoamine ketone transporters in human embryonic kidney cells [36]. Ketamine inhibits the uptake of catecholamines by neurons and the extracellular space, leading to a high adrenergic state and an increase in circulating norepinephrine concentration [35].
In addition, the dexmedetomidine is more effective than the traditional use of the benzodiazepine midazolam in reducing the cardiac stimulatory effects of ketamine and post-anesthesia delirium [36].
Activation of glutamate-independent pathways leads to an inward flow of Ca 2+ and mobilization into the calcium pool. The increase in Ca 2+ is concentrated in the cytoplasm of brain neurons, leading to the upregulation of dopamine and causing the adverse effects of pleasure and psychotropic drugs [41,42]. Ketamine can also act on cholinergic, nicotinic, and muscarinic receptors, thereby affecting the function of these receptors [36,43].
Progress of ketamine in cancer pain
The content of cancer pain includes somatic pain, visceral pain, neuropathic pain, sympathetic maintenance pain, inflammatory pain, etc [44]. Currently, there are conflicting reports on the treatment of cancer pain with ketamine. One meta-analysis suggests that ketamine may be a viable option for the treatment of refractory cancer pain [45].
However, there are also reports suggesting that ketamine is recommended as an adjuvant therapy for cancer pain relief in adult patients, and the quality of the evidence needs to be further investigated [46]. Currently, the main limitations of research in this area include the small number of included studies, small sample size, or inability to quantify the effects of ketamine.
Research progress on ketamine improving depression:
(1) NMDAR inhibition and AMPAR activation are the main pathways and mechanisms by which ketamine, its isomers and metabolites exert their antidepressant effects.
Although ketamine has shown strong clinical efficacy in the treatment of refractory depression. However, the underlying mechanisms remain unclear. It is currently believed that inhibition of NMDARs and activation of AMPARs are involved in ketamine-induced antidepressant effects [47]. There are also studies showing that the antidepressant effects of ketamine are related to BDNF/TrkB, mTORC1, and others [48,49]. Meanwhile, previous studies on hydroxydemethylamine and demethylamine have shown that they have antidepressant effects in rodents, mainly through the combined action of BDNF/TrkB. Inhibition of NMDAR and subsequent activation of AMPAR are the main pathways and mechanisms by which ketamine, its isomers and metabolites exert antidepressant effects [49].
In terms of antidepressant effects, ketamine can achieve disinhibition of glutamatergic neurons by blocking the NMDAR of inhibitory gamma-aminobutyric acid (GABA) neurons, leading to glutamate bursts and ultimately promoting synaptic protein synthesis and BDNF/TrkB release, resulting in antidepressant effects [50].
(2) Changes in metabolites in individuals treated with ketamine
Metabolomic biomarkers include metabolites from tissues, cells, and various body fluids. Metabolomics in treatment-resistant depression (TRD) has been shown to identify potential metabolite-based biomarkers for understanding the pathophysiology of the disease [51].
Previous investigators have examined metabolite changes in patients receiving ketamine/s-ketamine treatment and explored their relationship to ketamine response. The diagnosis of major depressive disorder (MDD) with suicidal tendency is associated with several metabolomic abnormalities. For example, plasma or serum metabolites (such as acylcarnitine, ATP, histidine, arginine, citrulline, kynurenine, and ornithine) are associated with abnormal oxidation and may serve as potential biomarkers for MDD [51-54]. The excessive activity of the HPA axis in MDD patients induces neuroendocrine-immune dysfunction, resulting in the secretion of glucocorticoids, which are important for energy balance in the body [55].
(3) Ketamine intervention alters synaptic plasticity
Similarly, ketamine has been shown to be effective in inducing long-term synaptic plasticity depressive behavior in several models, including chronic corticosterone (corticosterone) and repetitive constraint stress, anxiety disorders and PTSD, chronic pain, middle cerebral artery occlusion, and mild stress from chronic accidents [56-58].
The ketamine (2R, 6R) metabolite hydroxydemethylamine (2R, 6R-HNK) exhibits antidepressant activity and downstream sustained synaptic plasticity. Studies in mice [59,60], rats [61], and in vitro [62] have shown that 2R, 6R-HNK can maintain antidepressant efficacy and alter sustained synaptic plasticity [57].
To date, neurophysiological studies of ketamine and other RAADs have suggested a transient increase in postsynaptic membrane activity in glutamatergic neurons in the prefrontal cortex as the main possible mechanism. Whether selective inhibition of non-synaptic NMDARs can fully induce substantial synaptic reconstruction and enhance the efficacy of RAADs is still unknown.
(4) Ketamine alters cortical relations
Before/after ketamine administration, FC between bilateral thalamus and right middle frontal cortex (BA46) and between left thalamus and left anterior cingulate gyrus (BA8) is increased in patients. The FC between the right thalamus and the bilateral frontal poles (BA9) as well as between the right thalamus and the left paranasal gyrus (BA10) is reduced. However, after multiple comparisons, the above changes did not show statistical differences. Therefore, further research is needed to determine whether the changes in thalamic cortical connectivity induced by this drug are related to its antidepressant and anti-suicidal effects [63].
Knocking out STEP 61 in the medial prefrontal cortex (mPFC) can lead to abnormal expression of pTyr1472NR2B inside and outside of synapses and STEP 61 in the striatum of chronically stressed animals. Ketamine can correct these abnormalities while rapidly combating depression [64].
Resting state electroencephalography during baseline and 0.44 mg/kg dose of ketamine in 30 MDD patients. Use of a dynamic causal model to assess connectivity between the frontal and parietal lobes to fit the thalamic cortex model to the hierarchical connectivity nodes of the medial prefrontal cortex and superior parietal lobe.
Previous studies have shown that AMPA-mediated connectivity increases from the parietal lobe to the frontal lobe, while the GABA time constant is robustly decreased in the frontal lobe. These two parameters are relevant to the antidepressant properties of ketamine [65]. These results suggest that the antidepressant effect of ketamine is relevant to the acute frontal-parietal junction and GABA receptors.
(5) The antidepressant properties of ketamine and the TGF-β1/BDNF/TrkB pathway
Recently, hippocampal levels of TGF-β1 have been shown to parallel the depressive phenotype and cognitive impairment in animal models, and the clinical phenotype is low in response to "monoamine" antidepressants [66]. In the CSDS model, ketamine reduced the expression of TGF-β1 and its receptors in the prefrontal cortex and hippocampus of mice [67]. Similar results were confirmed in a mouse model of post-stroke depression [68].
Preservation of microglial function is particularly important for the antidepressant effect of ketamine [67]. In CSDS mice, intranasal administration of recombinant TGF-β1 activated BDNF/TrkB in microglia and produced antidepressant effects similar to those of ketamine [69]. Further research is needed to investigate the effects of regulating TGF-β1/BDNF/TrkB in microglia on the antidepressant effects of ketamine.
(6) Lateral habenular nucleus (LHb) involved in ketamine effects
1) Upregulation of β-CaMKII in the LHb is associated with clinical features of depression.
Quantitative proteomics shows that β-CaMKII is increased in the lateral tegmental nucleus of animal models, and antidepressants can downregulate its expression. In the lateral tegmental nucleus, increased β-CaMKII, but not α-CaMKII, significantly increased neuronal synaptic plasticity and resulted in severe depressive symptoms. Intervention with β-CaMKII-GluR1 can reverse these effects [70].
2) Ketamine has a selective inhibitory effect on NMDA receptors in specific brain regions in depression model mice [71].
Ketamine exhibits region-specific antidepressant effects by rapidly inhibiting NMDA receptor currents in LHb neurons of depression model mice. In depression model mice, LHb neurons showed high baseline activity and NMDA receptor open state, which made the effect of ketamine more significant in this area.
The study also found that the sensitivity of LHb neurons to ketamine can be exchanged by up-regulating CA1 neurons or down-regulating the activity of LHb neurons. This suggests that LHb is the primary brain target of ketamine's antidepressant effect, while other regions may be secondary targets. This distinction helps to design more precise and effective antidepressant treatments.
3) The fast-acting antidepressant ketamine rapidly alleviates depressive symptoms by blocking NMDA receptors in LHb [72].
In animal models of depression, LHb neurons showed significantly increased burst activity and theta wave synchronization, which were reversed by ketamine treatment. The burst activity of LHb neurons is dependent on NMDA receptors and T-type voltage-gated calcium channels (T-VSCCs), and blocking these channels can induce antidepressant effects in LHb. Ketamine reduces the burst activity of LHb neurons by blocking T-VSCC channels, thereby rapidly alleviating depressive symptoms. Systemic infusion of the T-VSCC blocker or local infusion of the T-VSCC antagonist mibefradil showed rapid antidepressant effects. The antidepressant properties of ketamine and T-VSCC blockers jointly block the activity of LHb neurons.
4) The role of LHb neuronal burst activity in depression, with Kir4.1 potassium channels in astrocytes is very important [73].
Research has shown that the activity of LHb neurons is significantly increased in depressed animals, driving depressive-like behavior that is the material basis of the rapid antidepressant ketamine.
The Kir4.1 potassium channel in LHb is significantly upregulated in a model of depression. Increased Kir4.1 current and protein levels are synchronized with the development of depressive-like symptoms, and this upregulation is associated with the membrane processes of astrocytes, which tightly surround neuronal cell bodies and synapses. By reducing extracellular potassium concentration or increasing Kir4.1 expression, the phenomenon of neuronal membrane potential hyperpolarization and increased burst activity observed in depression models can be simulated. These results suggest that Kir4.1 in LHb neuronal membrane and burst activity is a novel target for the treatment of depression.
5) A single injection of ketamine has sustained antidepressant effects via NMDAR traps in the LHb [74].
Ketamine can continuously inhibit the explosive activity of LHb and block NMDAR for up to 24 hours after systemic injection. The long-term inhibition of NMDAR is not achieved by endocytosis, but depends on the application of ketamine to NMDAR-dependent traps. This mechanism demonstrates the dynamic equilibrium regulation of NMDAR by ketamine at different plasma concentrations.
6) The ketamine's antidepressant effect and the autonomic nervous system
Several studies have suggested that the autonomic nervous system plays a critical role in human cardiovascular health and depression, an emotional disorder [75,76]. Skin electrical activity (EDA) is a promising and non-invasive tool to measure sympathetic nervous system activity associated with emotional and cognitive states.
EDA parameters are significantly negatively correlated with several pre-treatment depressive symptoms [77], suggesting that the level of pre-treatment depression is negatively associated to the decrease in pre-treatment sympathetic tension. In the CSDS model, splenectomy attenuated the PFC OXPHOS pathway (such as COX11, UQCR11, and ATP5e), which may help reduce depression. In addition, SRI-01138 (TGF-β1 receptor agonist) and subphrenic vagotomy can attenuate the antidepressant response of CSDS-sensitive mice to ketamine after splenectomy [78].
Different administration routes of ketamine
(1) Intravenous administration
When administered intravenously, a single dose of ketamine has a rapid onset and relatively short duration of action, with onset within 30 seconds, a distribution half-life of 10 minutes, and an elimination half-life of 2.0-3.0 hours. Ketamine has the characteristics of high lipid solubility and wide distribution [79].
A patient with metastatic cancer pain was started on ketamine (0.4 mg/kg.hr) infusion, and the patient's pain was completely relieved without significant adverse reactions. The stable dose of ketamine reduced the need for opioid medication by 61.4% [80].
(2) Intrathecal administration
A 2010 case report showed that intrathecal self-guided injection of the right-handed optical isomer of ketamine (S-ketamine) (20 mg/d) in combination with morphine and bupivacaine improved symptoms of refractory cancer pain without other adverse effects (hypertension and neurological dysfunction) [81]. Spinal co-administration of ketamine and morphine enhanced visceral and somatic anti-injury effects [82]. Intrathecal injection of ketamine attenuated severe refractory pain unresponsive to opioid escalation [83].
(3) Intranasal administration
1) During dressing changes, ketamine nasal drops can significantly reduce patients' pain scores without any significant adverse reactions [84]. And there were no adverse reactions associated with the use of ketamine in adult patients, including hypertension, tachycardia, skeletal muscle hyperactivity, increased upper respiratory secretions, nystagmus, and diplopia [85,86].
In studies of acute traumatic pain [87], renal colic [88], and neuropathic pain [89], intranasal S-ketamine can achieve similar analgesic effects with fewer side effects compared to the morphine treatment group. Compared with the gel lubricant group alone, the pain score caused by nasogastric tube insertion decreased in the ketamine gel lubricant group (P = 0.026) [90].
2) Brain-targeted nasal drug delivery device and its prospects for use in the nasal administration of ketamine
Although nasal drops, sprays and other forms have been widely used, because existing drug delivery devices cannot penetrate deep into the nasal olfactory area, some drugs are absorbed into the bloodstream as they pass through the mucous membrane of the respiratory tract, which is rich in capillaries and blood flow. This method can only deliver some drugs to the nasal olfactory area, while the other part of the drugs that enter the bloodstream and cannot cross the blood-brain barrier cannot function in the brain, resulting in drug waste. On the other hand, improper use of nasal drops can cause them to enter the throat, resulting in drug waste and discomfort for patients, especially in special populations such as infants and young children, which can lead to serious consequences such as throat spasms and choking.
In response to the above problems, we offer a nasal, brain-targeted drug delivery device (patented, CN202320800855.7) [91]. It includes a nasal plug, guide wire, occlusion balloon, drug delivery connector, storage box, pressure measurement connector, supply fan, exhaust fan and fan controller. Occlusion of the balloon can block the lower nasal passage and throat, allowing the atomised drug to be delivered into the nasal cavity by an air supply fan, remain in the nasal cavity for absorption by the olfactory area, and then enter the brain to play a targeted therapeutic role (Figure 1).
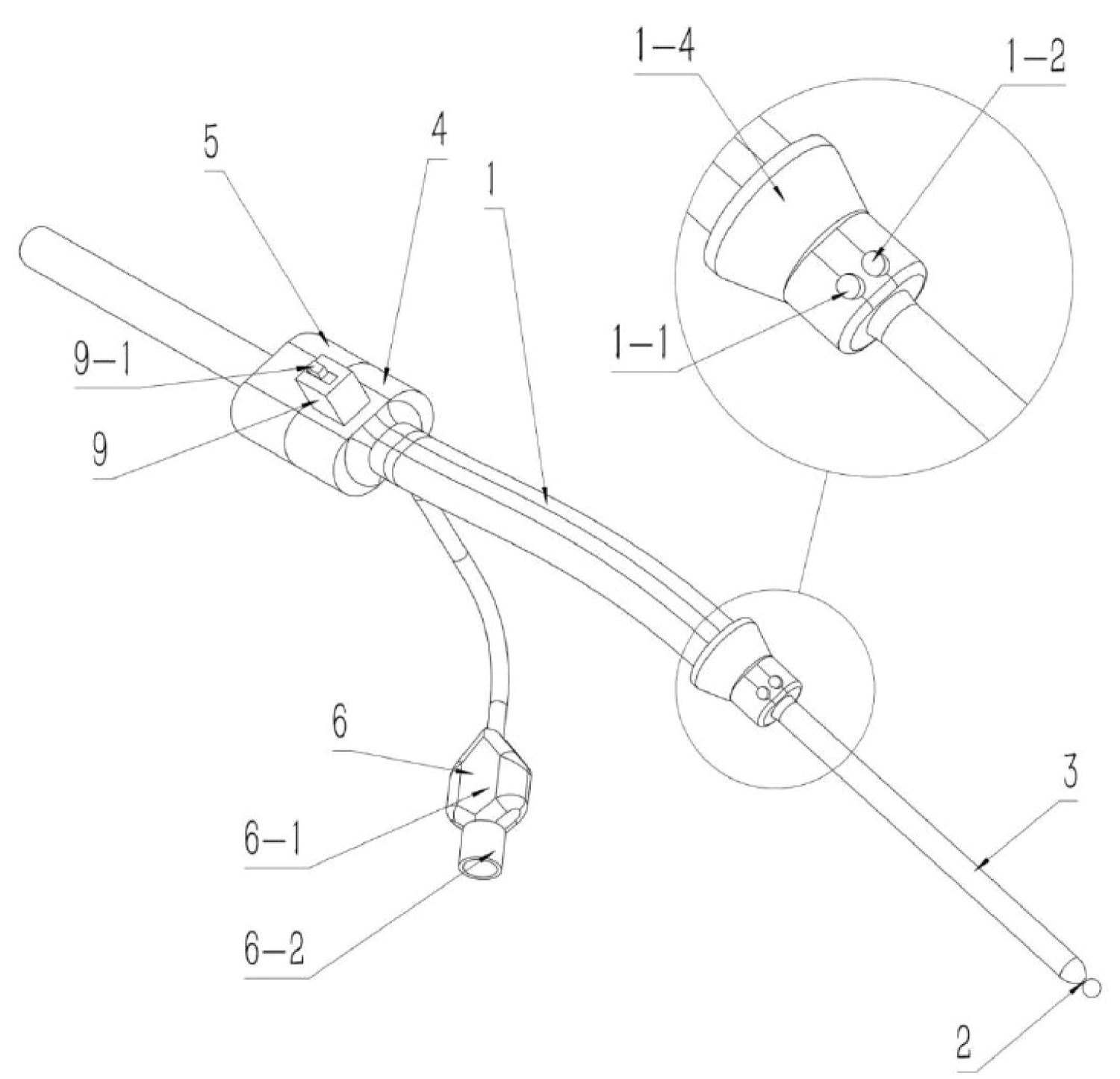
Figure 1: The nasal device for targeted drug delivery to the brain. The device comprises a nasal plug (1), a guide wire (2), a sealing air bag (3), a drug delivery connector (4), a storage box (5), a pressure measurement connector (6), an air supply fan (7), an exhaust fan (8) and a fan controller (9).
To date, there are no reports on the use of ketamine for nasal-brain targeted analgesia and antidepressant treatment. I believe that with the development of related research, targeted delivery of ketamine through the nose and brain will become a new drug delivery method for the treatment of cancer pain and depression.
(4) Other routes
In addition to the above routes of administration, ketamine can also be administered orally and rectally, but due to extensive hepatic first-pass elimination, the bioavailability of ketamine for treatment by these routes is low [79].
Ketamine and Tumor Recurrence
The perioperative procedures, including anesthesia, analgesia, surgical stress, blood transfusion, etc., may affect the proliferation and invasion ability of tumor cells, reduce the postoperative anti-tumor immune function of patients, and thus affect tumor metastasis and recurrence [92].
Immunoregulatory effects of ketamine
In clinical practice, balancing the relationship between immunosuppressive therapy and the risk of tumor recurrence is an important issue. Ketamine is a commonly used anesthetic and analgesic, and its immunomodulatory effects in the tumor environment have gradually attracted attention. Some studies indicated that it might affect immune responses in the tumor microenvironment through multiple mechanisms [92].
Studies have shown that NMDAR activation can induce lymphocyte activity, thereby affecting anti-tumor immune responses [91,92]. This suggests that ketamine may inhibit T cell proliferation and function, thereby affecting anti-tumor immune responses. NK cells are essential for innate immunity and are responsible for directly attacking tumor cells. Intraperitoneal injection of ketamine can inhibit the activity of NK cells, reduce their ability to kill tumor cells, and induce metastasis of lung cancer cells [93]. These effects suggest that ketamine and the adrenergic system may regulate each other [94]. In animal models, Ford, et al. confirmed that ketamine (intraperitoneal injection) can reduce NK cell activity and inhibit lung metastasis of breast cancer cells [95]. Zhou, et al. confirmed that both ketamine and morphine could inhibit the levels of IL-2 and IFN-γ in T lymphocytes [93,96].
Low-dose ketamine appears to have immunosuppressive effects by inhibiting pro-inflammatory cytokines, reducing T and NK cell activity, and promoting anti-inflammatory pathways. These effects may be beneficial in treating harmful conditions caused by excessive inflammation, but they may also carry risks, such as reduced immune surveillance for infections and tumors. Therefore, further attention needs to be paid to the use of different doses of ketamine in different clinical settings, particularly in patients with compromised immune function or those at risk of cancer recurrence.
The effect of perioperative ketamine use on immune regulation
As a vital component of the immune system, NK cells can recognize and destroy cancer cells and virus-infected cells [97-99]. During the perioperative period (before and after surgery), factors such as surgical pressure, anesthesia, and pain can lead to immune suppression and reduce NK cell activity [100].
A human clinical trial has confirmed that a single dose of ketamine (0.15 mg/kg) reduces postoperative inflammation but does not affect NK cell activity [100].
However, there are also reports to the contrary. Ketamine (0.25 mg/kg administered preoperatively; 0.05 mg/kg/h maintained during surgery) did not alter immune function in rectal cancer patients during surgery [101].
The differences in the above research results may be closely related to the dosage, timing, and tumor type of ketamine administration, which also suggests that ketamine regulates the diversity of the body's immune system [102-104].
To further investigate the above experiment, Zhang, et al. used different doses of s-ketamine (intravenous infusion, respectively) to study the effect of ketamine on immune-inflammation. They confirm that s-ketamine (0.25-0.75 mg/kg) can reduce the opioid dosages during the perioperative period, lower postoperative inflammatory responses and immunosuppression in breast cancer patients [105]. Further research with large samples and multiple centers is needed to confirm and determine the optimal dosage for use.
Direct effects of NMDA antagonists on cancer cell biology
Activation of NMDARs induces influx of Ca 2+ into cells, activating the CaMK II pathway, which can promote cellular processes that drive tumor proliferation [106]. Elevated intracellular calcium levels activate various signaling cascades, thereby promoting tumor growth, proliferation, death, and more [107,108].
Ketamine can downregulate key signaling molecules and reduce intracellular calcium levels, highlighting its potential anti-tumor effects. This inhibition of multiple pathways involved in cell survival, growth, angiogenesis and metastasis suggests that ketamine may be helpful for cancer treatment particularly in disrupting tumor growth and spread. Because of the critical role of calcium in cellular signaling, reducing intracellular calcium may impair many processes required for tumor cell survival, growth, and metastasis, including proliferation and migration [109,110].
Ketamine appears to promote lipid peroxidation and ferroptosis in cancer cells by regulating the lncPVT1/miR-214-3p/GPX4 axis. The accumulation of iron, lipid ROS and MDA, coupled with the decrease in GPX4 levels, weakens the cell's defense against oxidative stress, thereby inhibiting the survival ability of cancer cells. This mechanism highlights the potential pathway through which ketamine exerts its anticancer effects [110].
Some reports of perioperative ketamine promoting cancer recurrence
The immunomodulatory effect of ketamine in the tumor microenvironment may be twofold. Ketamine at anesthetic doses may inhibit the body's immune function, thereby promoting susceptibility to tumor metastasis [111-113]. Hofbauer, et al. confirmed that ketamine appears to inhibit LPS-induced granulocyte adhesion and migration and suppress inflammatory responses [111]. Ketamine has been shown to inhibit tissue damage induced by oxidative stress and to suppress macrophage activation and migration [112].
Compared with propofol, sodium thiopental, and halothane, inhibition of NK cell function by ketamine increases the susceptibility of tumors to metastasis because NK cells can recognize and destroy tumor cells. However, when beta-adrenergic blockers or low-dose immunostimulants are used as pretreatment, the effect of ketamine on promoting tumor metastasis can be significantly reduced [93,113].
Ketamine can enhance tumor growth by upregulating Bcl-2 in the MDA-MB-231 cell line [114]. High doses of S-ketamine and MK-801 promote the proliferation of pancreatic cancer cell lines, suggesting that further clinical research is needed to explore the impact of ketamine on cancer treatment [115].
NFATc2 may influence tumor progression by regulating the activity of other transcription factors critical for cancer cell proliferation and survival [116]. Ketamine (5 µM) inhibited the expression of NFAT in PaTu8988t cells within 24 and 48 hours. However, after 72 hours of ketamine stimulation, the expression of NFATc2 increased significantly, suggesting that long-term use may have unexpected effects on cancer cell signaling and progression [117].
Diagnosis and Treatment Status of Adverse Effects of Ketamine
Urinary toxicity
The main clinical manifestations of ketamine cystitis are urinary retention, urinary incontinence, hematuria, bladder wall thickening, bladder volume reduction, and other lower urinary tract symptoms [118]. Long-term use of ketamine may cause damage to the urinary system [119-121]. Although there are studies confirming that ketamine may cause damage to the urinary system, the research is still in the exploratory stage and includes the following aspects: Ketamine and its metabolite, ketamine, damage bladder barrier dysfunction; ketamine may increase bladder oxidative stress through mitochondrial and endoplasmic reticulum-related pathways, leading to bladder cell apoptosis and disruption of the urinary epithelial barrier; and related inflammatory mediators, etc. [118].
Hepatotoxicity
Ketamine is potentially hepatotoxic. Research has shown that ketamine is extensively metabolized in the liver to metabolites that can damage hepatic parenchymal cells and other liver cells [122]. Ketamine abuse may increase lipid peroxidation reactions, oxidative stress, and hepatocyte pyroptosis [121-123].
The sphincter of Oddi is a part of the biliary system responsible for regulating the flow of bile from the bile duct into the duodenum. Previous studies have shown that ketamine can relax biliary smooth muscle, relax the biliary tract, and inhibit gallbladder movement through central mechanisms by blocking NMDA receptors [124,125].
Therefore, hepatotoxicity of ketamine is a concern, especially for developing individuals who require ketamine for anesthesia. Research is ongoing to elucidate its mechanism and identify potential protective measures.
Cognitive impairment
Long-term use of ketamine may impair working memory, mainly by impairing visual memory, verbal memory, and response inhibition, as well as by significantly impairing selection ability and sustained attention [126-129]. Ketamine participates in the regulation of brain signaling pathways by blocking NMDA receptors and may indirectly upregulate D1 receptors in the lateral prefrontal cortex. This phenomenon persists even after chronic dopamine depletion [130]. The above suggests that excessive use of ketamine may affect the function of the lateral prefrontal cortex, resulting in impaired memory and cognitive function. Unfortunately, research on the treatment of chronic pain and depression is limited.
Although ketamine is known to cause some acute cognitive side effects during and shortly after administration, such as dissociation, sedation, or memory impairment, studies typically indicate that these effects are short-lived. Six weeks after infusion, cognitive function usually returns to baseline levels, and most patients have no significant long-term residual effects [13-133].
Research has shown that even shortly after administration (e.g., 40 minutes after injection), ketamine has minimal acute neurocognitive effects in many patients [132,133]. However, it is still important to monitor any potential cognitive or psychological effects on an ongoing basis, especially given the dissociative properties of ketamine. Over time, short-term infusions have also been shown to maintain the benefits of antidepressants without significantly impairing cognitive function. However, the potential safety of ketamine in long-term treatment regimens remains to be investigated.
Hemodynamic effects
Ketamine has significant effects on hemodynamics, with major hemodynamic effects including hemodynamic disorders, and increased myocardial oxygen demand. Ketamine is contraindicated in individuals with cardiovascular diseases, pulmonary heart disease, pulmonary arterial hypertension, intracranial pressure, or high intraocular pressure [134]. When used at lower doses (e.g., in psychiatric applications such as depression), hemodynamic effects are usually milder than with higher anesthetic doses [135].
Tolerance and dependence
People who use ketamine frequently may experience rapid development of craving, compulsive behavior, and tolerance. With frequent use of ketamine, tolerance develops rapidly, meaning that users must gradually increase their dose to achieve the same dissociation or euphoric effect [136]. This escalation can increase the cognitive impairment, bladder toxicity (ketamine bladder syndrome), and mental health problems, et al. [137].
Ketamine's unique effect on NMDARs, combined with its effect on the dopamine system, has led to these patterns of addiction. It has also been found that long-term or frequent use can lead to psychological dependence and physical health complications.
The side effects and safety risks of ketamine should be carefully considered before making a choice. Which isomer (R- and S-ketamine) causes more adverse effects, particularly cognitive and dissociative symptoms, in recreational or therapeutic use? Is one isomer more risk effective than the other for certain indications (e.g., depression, chronic pain)? Answering these questions will help guide the safe use of ketamine, particularly in clinical settings where its use is of particular importance. The occurrence and side effects of the two isomers in racemic mixtures of ketamine, as well as the effects of their metabolites, are still new areas of research that need to be explored.
It is recommended that large-scale clinical trials, particularly in patients with depression, be conducted to adequately understand the therapeutic potential and safety of ketamine. The trials should address several key issues, including: Multiple doses and treatment regimens, long-term follow-up of clinical treatment outcomes, monitoring of potential side effects, different patient populations, and comparative studies between R- and S-ketamine. Conducting these rigorous long-term studies will provide key data to optimize the use of ketamine in clinical practice, maximizing its therapeutic potential while minimizing its risks.
Research on Combination Use of Ketamine and Opioids
Compared to ketamine, R-ketamine has stronger sedative and analgesic properties. A large number of clinical trials abroad are also focusing on its use in combination with opioid medications. Ketamine as an adjuvant drug can improve the analgesic effect of morphine and reduce side effects at low doses.
Mechanistic studies of ketamine in combination with opioids
Perioperative use of opioids has been shown to cause hyperalgesia and hypersensitivity in patients: Morphine cannot reverse central neuronal sensitization. In addition to acting on opioid receptors, morphine peptides also excite NMDA receptors. High doses of morphine can cause diffuse sensory hypersensitivity that can be reversed by excitatory amino acid receptor antagonists [138]. Experimental studies have shown that right-handed ketamine can attenuate central sensitization and hyperalgesia and reduce postoperative patient tolerance to opioid medications [139]. In patients who have not used opioids prior to surgery, intraoperative use of ketamine at an anesthetic dose may reduce opioid consumption and pain [140].
Activated NMDARs participate in opioid tolerance: Opioid tolerance is an inevitable phenomenon involving activated NMDARs, nitric oxide and others [3,20]. Many neuronal factors are involved in the development of tolerance [141,142]: Reduced number of receptors, decoupling of receptor-effectors within cells, disruption of genome expression, adaptation of neuronal networks, etc.
In fact, it is the occupancy of opioid receptors that triggers tolerance, and as the number of occupied opioid receptors increases, tolerance becomes more likely. That is why tolerance is more likely to occur when opioid-resistant pain requires more morphine. NMDA receptor antagonists have antinociceptive properties in both neuropathic and nociceptive pain [143]. In addition, unlike morphine, these antagonists are effective even after administration. NMDA antagonists may attenuate morphine tolerance by modulating the effects of excitatory amino acids [143,144].
Neural regulation of periaqueductal gray (PAG) activity in the midbrain is the basis for the synergistic analgesic effect of morphine and ketamine: The periaqueductal gray (PAG) is critical in the integration of behavioral, somatic, and autonomic responses to threat, stress, and pain in animals [145,146]. Electroacupuncture can induce c-fos activation in the PAG [147], and the ventrolateral periaqueductal gray (vLPAG) is an important component mediating pain transmission [148,149]. The VLPAG is the basis of opioid analgesia. There is research confirming that neural regulation of VLPAG activity is the basis for the synergistic analgesic effect of morphine and ketamine.
The functional relationship between MOR and NMDAR is bidirectional. The functional cross-regulation between MOR and NMDAR in the PAG is regulated by protein kinase C (PKC)/PKA, and the postsynaptic association of these receptors is related to the transmission and regulation of nociceptive signals [149,150]. The bidirectional interaction between MOR and NMDAR in the PAG is related to the synaptic transmission of nociceptive signals by PKC/PKA [149,150]. Opioid drugs, including morphine, can disrupt the activity of MOR and NMDAR complexes that stimulate NMDAR, which can lead to abnormal regulation of the MOR pathway and opioid analgesic tolerance [151].
Perioperative combination with opioids to reduce perioperative opioid dose and prevent pain hypersensitivity
Many studies suggest that ketamine is best administered intravenously as an adjunct to opioids [152]. Although ketamine has the effect of sparing opioids, it has not been shown to reduce opioid-related adverse effects such as PONV, pruritus, and respiratory depression [153,154].
Future studies should focus on high-risk populations with opioid tolerance for acute postoperative pain and on the effects of low-dose ketamine for acute postoperative pain on long-term pain syndromes (after mastectomy, thoracotomy, and phantom limb pain).
Morphine-ketamine for refractory cancer pain
Refractory cancer pain in patients: In patients with terminal cancer, long-term overuse of morphine can lead to opioid tolerance and sensitization, and the dose of morphine must be continuously increased to achieve its original effect. This inevitably leads to serious side effects such as nausea, vomiting, and dyspnea [151-154].
Research has shown that low-dose ketamine can inhibit or reverse morphine tolerance and pain sensitization, enhance the analgesic effect of opioid drugs, thereby reducing the dose of morphine and alleviating its adverse reactions [155]. When morphine is tolerated, ketamine can reduce the dose of the intrathecal analgesic morphine by 50% [138,156].
In a randomized, double-blind trial, 48 patients with advanced cancer received epidural pain management when systemic opioid and NSAID treatment was ineffective or intolerable [157]. The results showed that low-dose ketamine or neostigmine significantly prolonged the analgesic effect in patients, whereas mid-dose midazolam did not have this effect.
Two cancer patients [158] experienced decreased pain sensitivity and improved quality of life after receiving combined epidural ketamine, morphine, and bupivacaine. After receiving adjuvant ketamine, the patient had decreased pain sensitivity and no serious adverse events. Low-dose ketamine prolonged satisfactory analgesic effects and partially restored patients' quality of life when conventional treatment failed. The combination of ketamine with morphine and bupivacaine may prolong analgesia and reduce tolerance to morphine.
In one study [159], researchers evaluated the efficacy and safety of 5 days of continuous intravenous infusion (CIVI) and 5 days of stepwise titration of ketamine in the treatment of advanced cancer patients with refractory cancer pain. The results showed that ketamine CIVI may be a useful tool in the treatment of refractory cancer patients.
Researchers [160] evaluated the efficacy of different doses of intranasal ketamine (10, 30, and 50 milligrams) in treating refractory cancer pain. All doses of intranasal ketamine were well tolerated with short-term side effects, and no serious adverse events related to the study drug were reported. Pain relief began within 15 minutes in most patients and continued throughout the study. The study showed that the exposure level of ketamine is consistent with the clearance rate, and the bioavailability of intranasal administration is approximately 50%.
Katzenschlager, et al. [161] confirmed that intrathecal injection of S (+)-ketamine is an effective method for treating chronic cancer-related neuropathic pain. However, its application to nerve axons carries potential risks in the absence of sufficient safety data. Current research findings has generated controversy regarding the neurotoxicity of the drug and further research is needed to comprehensively evaluate the long-term safety of intrathecal administration of S(+)-ketamine and its rationale in clinical practice.
In vitro study of the effect of the interaction between ketamine and morphine on T cells: There is currently limited research on whether morphine and ketamine have an effect on the immune system in refractory cancer pain. Morphine, an exogenous opioid peptide, exerts potent analgesic effects by binding to opioid peptide receptors. Opioids can activate NMDA receptors and lead to tolerance. Ketamine has an affinity for opioid receptors that is only 1/10-1/20 of its affinity for NMDA receptors, but it can also promote the release of endogenous opioid peptides and stimulate opioid receptors, thereby exerting some analgesic effect [149-151]. Ketamine can also act on nicotinic and muscarinic acetylcholine receptors, block sodium channels, and interact with opioid receptors and calcium ion channels [162].
In addition, ketamine can inhibit the reabsorption of monoamine neurotransmitters (dopamine, norepinephrine, and serotonin et al.). This activates or enhances the central pain inhibitory system, resulting in analgesic effects [163,164]. The sites of action of morphine and ketamine as two analgesics overlap, and their effects on the body's immunity may interact. Morphine and ketamine can simultaneously act on opioid receptors and exert immunosuppressive effects through multiple pathways. However, with respect to NMDA receptors, morphine exerts an excitatory effect while ketamine exerts an antagonistic effect. There are no relevant reports on which of the two has an advantage, either immunosuppression or immunopotentiation.
Although ketamine itself has immunosuppressive effects, it is currently the least immunosuppressive drug among general anesthetics. While morphine combined with low-dose ketamine provides adequate pain relief, it has been suggested that it may alleviate the immunosuppressive state of refractory cancer pain patients.
The combination of morphine and ketamine can reduce the CD4+ percentage, and CD4+/CD8+ levels are detected through the JAK3/STAT5 pathway for IFN-Y, IL-2, and IL-17 [165]. This study confirms that this combination therapy can improve tumor pain and suppress the immune system. However, there are currently few studies on this topic, both nationally and internationally, and this conclusion still requires a large amount of experimental data to support.
Efficacy and safety of ketamine as adjuvant opioid therapy for cancer pain
As an analgesic adjunct to opioids, the quality of evidence for ketamine research is low, often resulting in small sample sizes and a high risk of bias [166]. The study by Hardy, et al. suggests that ketamine does not significantly improve analgesia compared with placebo, and the incidence of adverse events (including hallucinations and cognitive impairment) in the ketamine group is almost twice that of the placebo group [167].
The three studies used different routes and doses of ketamine, including intrathecal administration [168], intravenous injection [169], and subcutaneous injection [167]. Different routes and doses may affect the efficacy and safety of ketamine, but current studies are insufficient to determine the optimal route and dose.
The reasons for the inconsistent research results with ketamine in the past can be explained as follows. First, most studies have been conducted in heterogeneous patient populations, ranging from those undergoing anticancer treatment to patients with advanced cancer. Second, most studies lack a clear definition of pain in refractory cancer or include patients with low pain intensity. When high doses of opioids are required, opioid tolerance is evident and the likelihood of a neurologic component is high.
At this point, it may be more necessary to combine the use of ketamine for adjuvant analgesia. Third, many trials, including phase 3 trials, have used administration methods such as pill injection or continuous subcutaneous infusion (CSCI). The dose injection method is very convenient, but some people are concerned that it may cause serious adverse events (AEs) and AEs due to skin irritation, so the CSCI method is not recommended. The progressive dose titration method of continuous intravascular infusion (CIVI), although inconvenient for maintaining venous access, has the advantage of safely starting at a lower dose and increasing to an adequate dose as needed.
Current research data are insufficient to confirm that ketamine can be used as an opioid adjuvant in the treatment of cancer pain. Future research should pay particular attention to the interactions between ketamine and specific opioid drugs, as well as the optimal dosage, therapeutic efficacy, and safety evaluation of ketamine in different cancer types and treatment stages.
Research Progress in the Discovery of Ketamine in Patients with CPD
The current research shows prospectively that ketamine can effectively treat refractory cancer pain and depression. The treatment of cancer pain in combination with depression is challenging. The comorbidity of depression and cancer pain remains a challenging issue. Ketamine's antidepressant effects may also be useful for CPD.
CPD, as an important factor affecting the condition and prognosis of cancer patients, is an urgent problem to be solved in cancer treatment. The incidence rate of depression in patients with advanced cancer is high, reaching 16.5%, and severely affects quality of life and survival [170]. Conventional antidepressants have limited efficacy and slow onset of action in advanced cancer patients and are not suitable for all patients.
A 39-year-old female patient with stage IVB cervical cancer, intractable pain, and suicidal ideation. After receiving an injection of ketamine (0.5 mg/kg, IV), her depressive symptoms rapidly improved, suicidal ideation disappeared, and pain was relieved. The patient continued to show significant improvement with subsequent treatment, demonstrating that ketamine can enhance the efficacy of conventional antidepressants [170]. This study highlights that ketamine may be an alternative for cancer pain patients who have poor efficacy or intolerance to conventional antidepressants.
A 64-year-old man with advanced thyroid cancer was hospitalized for severe neuropathic pain and major depression. After an intravenous injection of ketamine, the patient's pain, depression symptoms, and pain score decreased significantly [171].
A patient with metastatic ovarian cancer showed rapid and sustained improvement in depressive symptoms and pain reduction after treatment with ketamine (1 mg/kg, intramuscular injection, repeated doses at weekly intervals) [172].
A 50-year-old patient with metastatic prostate cancer was admitted for chronic nausea, vomiting, and severe orthostatic hypotension. The patient was diagnosed with major depression and had a poor response to existing medications such as venlafaxine. In the palliative care unit, the patient received ketamine "burst therapy" (0.5 mg/kg, intravenous drip over 60 minutes). After the first treatment, the patient's mood improved, but the effect was not sustained. The effect of the second treatment was more short-lived, although there was a mild side effect of hallucinations [173].
Conclusion
Currently, there is a lack of empirical evidence for the treatment of chronic pain-related depression with ketamine. Most of the available data are case reports, which may not be sufficient to draw definitive conclusions. The available literature suggests that ketamine may serve as an effective alternative for people with cancer pain who have poor efficacy or intolerance to conventional antidepressants. Nevertheless, further clinical trials are needed to determine the safety and long-term effects of ketamine, particularly in patients.
Ketamine may provide an initial antidepressant effect in patients with CPD (Figure 2). However, the sustainability of its therapeutic effects is limited, and the optimal dosage and administration regimen need further verification. Furthermore, it is of interest to assess its impact on patients' emotional state and suicide risk in the context of cancer pain management.
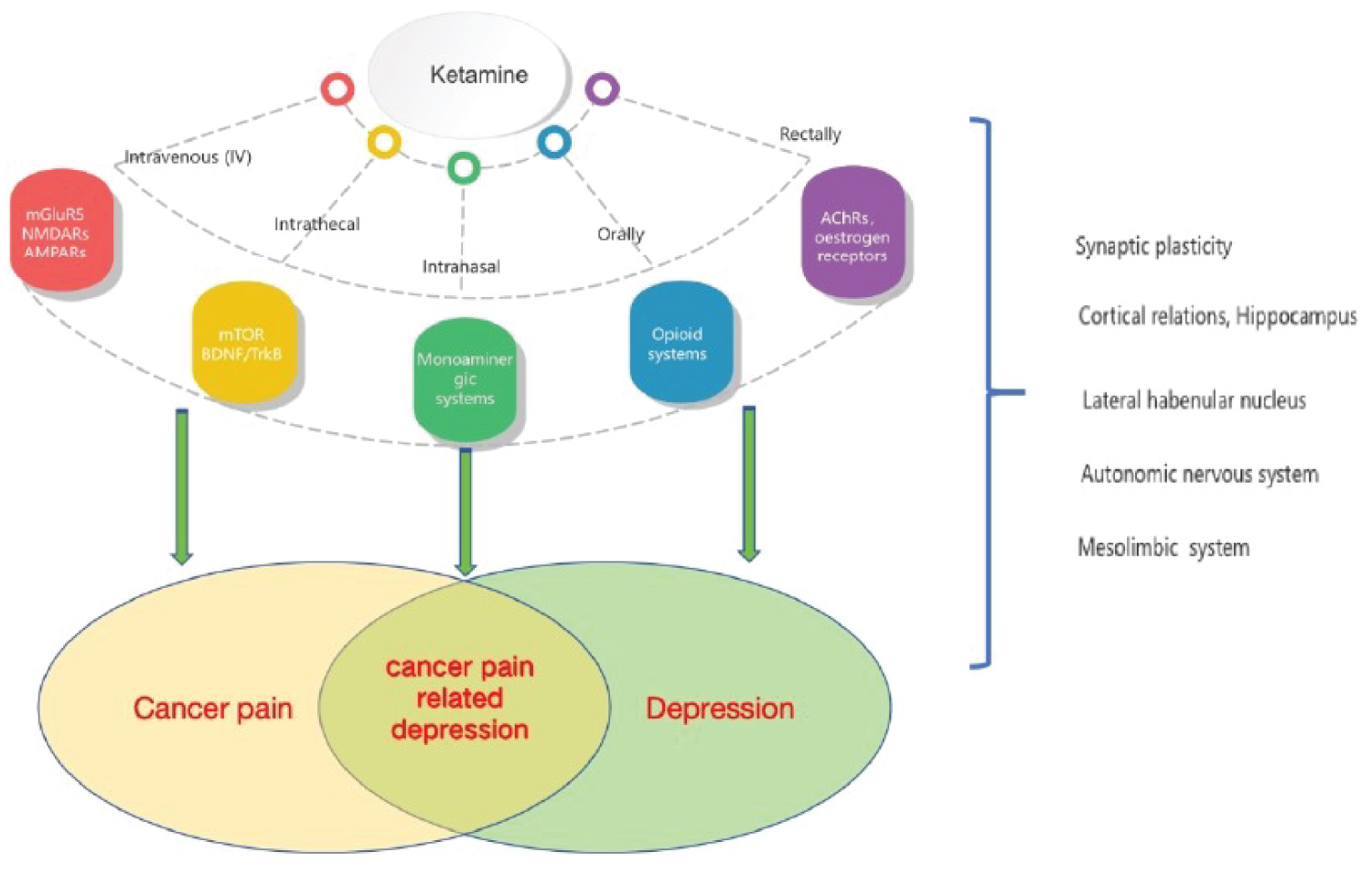
Figure 2: The therapeutic effects of ketamine on cancer pain related depression.
Funding
National Natural Science Foundation of China (Project Approval Number: 81200858).
National Natural Science Foundation of China (Project Approval Number: 31100801).
Jiangsu Province "333 High-level Talent Training Project" [Certificate No.: (2022) No. 3-10-007].
Conflict of Interests Statement
The author is the inventor of the device mentioned in this review. (A brain-targeted nasal drug delivery device: CN202320800855.7).
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021 May;71(3):209-249.
- Han B, Zheng R, Zeng H, Wang S, Sun K, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent 2024;4(1):47-53.
- Xia S, Zhu Y, Luo L, Wu W, Ma L, et al. Prognostic value of depression and anxiety on colorectal cancer-related mortality: a systematic review and meta-analysis based on univariate and multivariate data. Int J Colorectal Dis 2024;39(1):45.
- Gao J, Wu G, Mao E, Zhao H. Auricular acupuncture for breast cancer-related depression: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 2020;99(45):e22870.
- Abdul Razak N, Azhar ZI, Ismail Z, Mohd Azman ZA, Abdul Manap SA, et al. Impact of pilates exercise on quality of life, functional capacity, cancer-related fatigue, depression and salivary cortisol of colorectal cancer survivors: A quasi-experimental study. Asian Pac J Cancer Prev 2024;25(8):2895-2904.
- Zhu Q, Yang L, Yang H, Han Y, Chen Y, et al. Quercetin alleviates the progression of breast cancer-related depression via inhibiting the pyroptosis and promoting the immune response. Mediators Inflamm 2022;2022:8011988.
- Lobefaro R, Rota S, Porcu L, Brunelli C, Alfieri S, et al. Cancer-related fatigue and depression: A monocentric, prospective, cross-sectional study in advanced solid tumors. ESMO Open 2022;7(2):100457.
- Hong H, Ji M, Lai D. Chronic stress effects on tumor: Pathway and mechanism. Front Oncol 2021;11:738252.
- Jiang Y, Hu Y, Yang Y, Yan R, Zheng L, et al. Tong-Xie-Yao-Fang promotes dendritic cells maturation and retards tumor growth in colorectal cancer mice with chronic restraint stress. J Ethnopharmacol 2024;319(Pt 1):117069.
- Schmidt ME, Maurer T, Behrens S, Seibold P, Obi N, et al. Cancer-related fatigue: Towards a more targeted approach based on classification by biomarkers and psychological factors. Int J Cancer 2024;154(6):1011-1018.
- Qin N, Yi S, Dai C, Liu X, Duan Y, et al. Associations of serum cortisol and inflammatory marker features with depression and anxiety in young women with gynecologic cancer. Support Care Cancer 2023;31(12):674.
- Chen SJ, Chang CH, Chen KC, Liu CY. Association between depressive disorders and risk of breast cancer recurrence after curative surgery. Medicine (Baltimore) 2016;95(33):e4547.
- Alves I, Moreira AP, Sousa T, Teles P, Fernandes CS, et al. Exergame-based rehabilitation for cancer patients undergoing abdominal surgery: Effects on pain, anxiety, depression, and fatigue-A pilot study. Eur J Oncol Nurs 2024;72:102665.
- Ye Y, Zeng K, Qin L, Luo J, Liu S, et al. Differential characteristics of fatigue-pain-sleep disturbance-depression symptom cluster and influencing factors of patients with advanced cancer during treatment: A latent class analysis. Cancer Nurs 2024.
- Kai-hoi Sze F, Wong E, Lo R, Woo J. Do pain and disability differ in depressed cancer patients? Palliat Med 2000;14(1):11-17.
- Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology 2001;10(1):19-28.
- Stommel M, Given BA, Given CW. Depression and functional status as predictors of death among cancer patients. Cancer 2002;94(10):2719-2727.
- Laird BJ, Boyd AC, Colvin LA, Fallon MT. Are cancer pain and depression interdependent? A systematic review. Psychooncology 2009;18(5):459-464.
- Lloyd-Williams M, Dennis M, Taylor F. A prospective study to determine the association between physical symptoms and depression in patients with advanced cancer. Palliat Med 2004;18(6):558-563.
- Chen LP, Dai HY, Dai ZZ, Xu CT, Wu RH. Anterior cingulate cortex and cerebellar hemisphere neurometabolite changes in depression treatment: A 1H magnetic resonance spectroscopy study. Psychiatry Clin Neurosci 2014;68(5):357-364.
- Zimmerman L, Story KT, Gaston-Johansson F, Rowles JR. Psychological variables and cancer pain. Cancer Nurs 1996;19(1):44-53.
- Remy F, Frankenstein UN, Mincic A, Tomanek B, Stroman PW. Pain modulates cerebral activity during cognitive performance. Neuroimage 2003;19(3):655-664.
- Poulin TG, Jaworska N, Stelfox HT, Fiest KM, Moss SJ. Clinical practice guideline recommendations for diagnosis and management of anxiety and depression in hospitalized adults with delirium: A systematic review. Syst Rev 2023;12(1):174.
- Simon GE, Moise N, Mohr DC. Management of depression in adults: A review. JAMA 2024;332(2):141-152.
- Adult Cancer Pain. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Version 1.2023-March 7, 2023.
- Adult Cancer Pain. NCCN clinical practice guidelines in oncology (NCCN Guidelines® ). Version 2.2024 - March 11, 2024
- Jahn H. Steroid-synthesis inhibition in depression: A good idea? Lancet Psychiatry 2016;3(2):92-93.
- Whale R, Fialho R, Field AP, Campbell G, Tibble J, et al. Factor analyses differentiate clinical phenotypes of idiopathic and interferon-alpha-induced depression. Brain Behav Immun 2019;80:519-524.
- Kalin NH. The critical relationship between anxiety and depression. Am J Psychiatry 2020;177(5):365-367.
- Kalin NH. Stress, heritability, and genetic factors influencing depression, PTSD, and suicidal behavior. Am J Psychiatry 2023;180(10):699-702.
- Kumar R, LeMahieu AM, Stan MN, Seshadri A, Ozerdem A, et al. The association between thyroid stimulating hormone and depression: A historical cohort study. Mayo Clin Proc 2023;98(7):1009-1020.
- Mainio A, Hakko H, Niemela A, Koivukangas J, Rasanen P. Gender difference in relation to depression and quality of life among patients with a primary brain tumor. Eur Psychiatry 2006;21(3):194-199.
- Ronaldson A, Arias de la Torre J, Sima R, Ashworth M, Armstrong D, et al. Prospective associations between depression and risk of hospitalisation for infection: Findings from the UK Biobank. Brain Behav Immun 2022;102:292-298.
- Zhou JS, Peng GF, Liang WD, et al. Recent advances in the study of anesthesia-and analgesia-related mechanisms of S-ketamine. Front Pharmacol. 2023;14:1228895.
- Zhang Y, Ye F, Zhang T, Lv S, Zhou L, et al. Structural basis of ketamine action on human NMDA receptors. Nature 2021;596(7871):301-305.
- Peltoniemi MA, Hagelberg NM, Olkkola KT, Saari TI. Ketamine: A review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin Pharmacokinet 2016;55(9):1059-1077.
- Bieber M, Schwerin S, Kreuzer M, Klug C, Henzler M, et al. s-ketamine enhances thalamocortical and corticocortical synaptic transmission in acute murine brain slices via increased AMPA-receptor-mediated pathways. Front Syst Neurosci 2022;16:1044536.
- Tian F, Lewis LD, Zhou DW, Balanza GA, Paulk AC, et al. Characterizing brain dynamics during ketamine-induced dissociation and subsequent interactions with propofol using human intracranial neurophysiology. Nat Commun 2023;14(1):1748.
- Smith DJ, Bouchal RL, deSanctis CA, Monroe PJ, Amedro JB, et al. Properties of the interaction between ketamine and opiate binding sites in vivo and in vitro. Neuropharmacology 1987;26(9):1253-1260.
- Hustveit O, Maurset A, Oye I. Interaction of the chiral forms of ketamine with opioid, phencyclidine, sigma and muscarinic receptors. Pharmacol Toxicol 1995;77(6):355-359.
- Kornhuber J, Mack-Burkhardt F, Kornhuber ME, Riederer P. [3H] MK-801 binding sites in post-mortem human frontal cortex. Eur J Pharmacol 1989;162(3):483-490.
- Kapur S, Seeman P. NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia. Mol Psychiatry 2002;7(8):837-844.
- Drummond JC, Brebner J, Galloon S, Young PS. A randomized evaluation of the reversal of ketamine by physostigmine. Can Anaesth Soc J 1979;26(4):288-295.
- Jiao J, Fan J, Zhang Y, Chen L. Efficacy and safety of ketamine to treat cancer pain in adult patients: A systematic review. J Pain Symptom Manage 2024;67(3):e185-e210.
- Bredlau AL, Thakur R, Korones DN, Dworkin RH. Ketamine for pain in adults and children with cancer: A systematic review and synthesis of the literature. Pain Med 2013;14(10):1505-1517.
- Bell RF, Eccleston C, Kalso EA. Ketamine as an adjuvant to opioids for cancer pain. Cochrane Database Syst Rev 2017;6(6):CD003351.
- Yang C, Yang J, Luo A, Hashimoto K. Molecular and cellular mechanisms underlying the antidepressant effects of ketamine enantiomers and its metabolites. Transl Psychiatry 2019;9(1):280.
- Dandekar MP, Peng T, McPherson DD, Quevedo J, Soares JC, et al. Intravenous infusion of xenon-containing liposomes generates rapid antidepressant-like effects. Prog Neuropsychopharmacol Biol Psychiatry 2018;86:140-149.
- Tan Y, Fujita Y, Qu Y, Chang L, Pu Y, et al. Phencyclidine-induced cognitive deficits in mice are ameliorated by subsequent repeated intermittent administration of (R)-ketamine, but not (S)-ketamine: Role of BDNF-TrkB signaling. Pharmacol Biochem Behav 2020;188:172839.
- Kowalczyk M, Kowalczyk E, Kwiatkowski P, Lopusiewicz L, Sienkiewicz M, et al. Ketamine-new possibilities in the treatment of depression: A narrative review. Life (Basel) 2021;11(11).
- Kumar R, Nunez NA, Joshi N, Joseph B, Verde A, et al. Metabolomic biomarkers for (R, S)-ketamine and (S)-ketamine in treatment-resistant depression and healthy controls: A systematic review. Bipolar Disord 2024;26(4):321-330.
- Milaneschi Y, Arnold M, Kastenmuller G, Dehkordi SM, Krishnan RR, et al. Mood disorders precision medicine C. Genomics-based identification of a potential causal role for acylcarnitine metabolism in depression. J Affect Disord 2022;307:254-263.
- Allen J, Romay-Tallon R, Brymer KJ, Caruncho HJ, Kalynchuk LE. Mitochondria and mood: Mitochondrial dysfunction as a key player in the manifestation of depression. Front Neurosci 2018;12:386.
- Rotroff DM, Corum DG, Motsinger-Reif A, Fiehn O, Bottrel N, et al. Metabolomic signatures of drug response phenotypes for ketamine and esketamine in subjects with refractory major depressive disorder: New mechanistic insights for rapid acting antidepressants. Transl Psychiatry 2016;6(9):e894.
- Farrell C, Doolin K, N OL, Jairaj C, Roddy D, et al. DNA methylation differences at the glucocorticoid receptor gene in depression are related to functional alterations in hypothalamic-pituitary-adrenal axis activity and to early life emotional abuse. Psychiatry Res 2018;265:341-348.
- Krzystyniak A, Baczynska E, Magnowska M, Antoniuk S, Roszkowska M, et al. Prophylactic ketamine treatment promotes resilience to chronic stress and accelerates recovery: Correlation with changes in synaptic plasticity in the CA3 subregion of the hippocampus. Int J Mol Sci 2019;20(7).
- Ruan Y, Yuan R, He J, Jiang Y, Chu S, et al. New perspective on sustained antidepressant effect: Focus on neurexins regulating synaptic plasticity. Cell Death Discov 2024;10(1):205.
- Cavalleri L, Merlo Pich E, Millan MJ, Chiamulera C, Kunath T, et al. Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Mol Psychiatry 2018;23(4):812-823.
- Yao N, Skiteva O, Zhang X, Svenningsson P, Chergui K. Ketamine and its metabolite (2R, 6R)-hydroxynorketamine induce lasting alterations in glutamatergic synaptic plasticity in the mesolimbic circuit. Mol Psychiatry 2018;23(10):2066-2077.
- Fukumoto K, Fogaca MV, Liu RJ, Duman C, Kato T, et al. Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2R, 6R)-hydroxynorketamine. Proc Natl Acad Sci U S A 2019;116(1):297-302.
- Chou D, Peng HY, Lin TB, Lai CY, Hsieh MC, et al. (2R, 6R)-hydroxynorketamine rescues chronic stress-induced depression-like behavior through its actions in the midbrain periaqueductal gray. Neuropharmacology 2018;139:1-12.
- Collo G, Cavalleri L, Chiamulera C, Merlo Pich E. (2R, 6R)-Hydroxynorketamine promotes dendrite outgrowth in human inducible pluripotent stem cell-derived neurons through AMPA receptor with timing and exposure compatible with ketamine infusion pharmacokinetics in humans. Neuroreport 2018;29(16):1425-1430.
- Tu PC, Chang WC, Su TP, Lin WC, Li CT, et al. Thalamocortical functional connectivity and rapid antidepressant and antisuicidal effects of low-dose ketamine infusion among patients with treatment-resistant depression. Mol Psychiatry 2024.
- Wang K, Tan X, Ding KM, Feng XZ, Zhao YY, et al. Dynamic regulation of phosphorylation of NMDA receptor GluN2B subunit tyrosine residues mediates ketamine rapid antidepressant effects. Pharmacol Res 2024;205:107236.
- Sumner RL, McMillan RL, Forsyth A, Muthukumaraswamy SD, Shaw AD. Neurophysiological evidence that frontoparietal connectivity and GABA-A receptor changes underpin the antidepressant response to ketamine. Transl Psychiatry 2024;14(1):116.
- Caraci F, Spampinato SF, Morgese MG, Tascedda F, Salluzzo MG, et al. Neurobiological links between depression and AD: The role of TGF-beta1 signaling as a new pharmacological target. Pharmacol Res 2018;130:374-384.
- Zhang K, Yang C, Chang L, Sakamoto A, Suzuki T, et al. Essential role of microglial transforming growth factor-beta1 in antidepressant actions of (R)-ketamine and the novel antidepressant TGF-beta1. Transl Psychiatry 2020;10(1):32.
- Zhang Y, Xie B, Yuan Y, Zhou T, Xiao P, et al. (R, S)-ketamine promotes striatal neurogenesis and sensorimotor recovery through improving poststroke depression-mediated decrease in atrial natriuretic peptide. Biol Psychiatry Glob Open Sci 2021;1(2):90-100.
- Wei Y, Chang L, Hashimoto K. Intranasal administration of transforming growth factor-beta1 elicits rapid-acting antidepressant-like effects in a chronic social defeat stress model: A role of TrkB signaling. Eur Neuropsychopharmacol 2021;50:55-63.
- Li K, Zhou T, Liao L, Yang Z, Wong C, et al. betaCaMKII in lateral habenula mediates core symptoms of depression. Science 2013;341(6149):1016-1020.
- Chen M, Ma S, Liu H, Dong Y, Tang J, et al. Brain region-specific action of ketamine as a rapid antidepressant. Science 2024;385(6709):eado7010.
- Yang Y, Cui Y, Sang K, Dong Y, Ni Z, et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 2018;554(7692):317-322.
- Cui Y, Yang Y, Ni Z, Dong Y, Cai G, et al. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 2018;554(7692):323-327.
- Ma S, Chen M, Jiang Y, Xiang X, Wang S, et al. Sustained antidepressant effect of ketamine through NMDAR trapping in the LHb. Nature 2023;622(7984):802-809.
- Bellato A, Sesso G, Milone A, Masi G, Cortese S. Systematic review and meta-analysis: Altered autonomic functioning in youths with emotional dysregulation. J Am Acad Child Adolesc Psychiatry 2024;63(2):216-230.
- Kovacova V, Macejova A, Tonhajzerova I, Visnovcova Z, Ferencova N, et al. Effect of acute ketamine treatment on sympathetic regulation indexed by electrodermal activity in adolescent major depression. Pharmaceuticals (Basel) 2024;17(3).
- Fernandez D, Ros L, Sanchez-Reolid R, Ricarte JJ, Latorre JM. Effectiveness of the level of personal relevance of visual autobiographical stimuli in the induction of positive emotions in young and older adults: Pilot study protocol for a randomized controlled trial. Trials 2020;21(1):663.
- Chang L, Wei Y, Qu Y, Zhao M, Zhou X, et al. Role of oxidative phosphorylation in the antidepressant effects of arketamine via the vagus nerve-dependent spleen-brain axis. Neurobiol Dis 2024;199:106573.
- Zgaia AO, Irimie A, Sandesc D, Vlad C, Lisencu C, et al. The role of ketamine in the treatment of chronic cancer pain. Clujul Med 2015;88(4):457-461.
- Amin P, Roeland E, Atayee R. Case report: Efficacy and tolerability of ketamine in opioid-refractory cancer pain. J Pain Palliat Care Pharmacother 2014;28(3):233-242.
- Sato C, Okabe T, Nakanishi K, Sakamoto A. A case of cancer pain management by long-term intrathecal PCA. J Nippon Med Sch 2010;77(6):333-337.
- Miyamoto H, Saito Y, Kirihara Y, Hara K, Sakura S, et al. Spinal coadministration of ketamine reduces the development of tolerance to visceral as well as somatic antinociception during spinal morphine infusion. Anesth Analg 2000;90(1):136-141.
- Fisher K, Coderre TJ, Hagen NA. Targeting the N-methyl-D-aspartate receptor for chronic pain management. Preclinical animal studies, recent clinical experience and future research directions. J Pain Symptom Manage 2000;20(5):358-373.
- Page N, Nirabhawane V. Intranasal ketamine for the management of incidental pain during wound dressing in cancer patients: A pilot study. Indian J Palliat Care 2018;24(1):58-60.
- Newton A, Fitton L. Intravenous ketamine for adult procedural sedation in the emergency department: A prospective cohort study. Emerg Med J 2008;25(8):498-501.
- Niesters M, Martini C, Dahan A. Ketamine for chronic pain: Risks and benefits. Br J Clin Pharmacol 2014;77(2):357-367.
- Shimonovich S, Gigi R, Shapira A, Sarig-Meth T, Nadav D, et al. Intranasal ketamine for acute traumatic pain in the Emergency Department: A prospective, randomized clinical trial of efficacy and safety. BMC Emerg Med 2016;16(1):43.
- Farnia MR, Jalali A, Vahidi E, Momeni M, Seyedhosseini J, et al. Comparison of intranasal ketamine versus IV morphine in reducing pain in patients with renal colic. Am J Emerg Med 2017;35(3):434-437.
- Huge V, Lauchart M, Magerl W, Schelling G, Beyer A, et al. Effects of low-dose intranasal (S)-ketamine in patients with neuropathic pain. Eur J Pain 2010;14(4):387-394.
- Nejati A, Golshani K, Moradi Lakeh M, Khashayar P, Moharari RS. Ketamine improves nasogastric tube insertion. Emerg Med J 2010;27(8):582-585.
- Cheng W, Yin Q, Wang J-F, Zou Z. A brain-targeted nasal drug delivery device: CN202320800855.7 [P] 2023-10-03.
- Juric A, Lovakovic BT, Zandona A, Rasic D, Cesi M, et al. The effects of ketamine on viability, primary DNA damage, and oxidative stress parameters in HepG2 and SH-SY5Y cells. Arh Hig Rada Toksikol 2023;74(2):106-114.
- Melamed R, Bar-Yosef S, Shakhar G, Shakhar K, Ben-Eliyahu S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: Mediating mechanisms and prophylactic measures. Anesth Analg 2003;97(5):1331-1339.
- Shakhar G, Ben-Eliyahu S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J Immunol 1998;160(7):3251-3258.
- Forget P, Collet V, Lavand'homme P, De Kock M. Does analgesia and condition influence immunity after surgery? Effects of fentanyl, ketamine and clonidine on natural killer activity at different ages. Eur J Anaesthesiol 2010;27(3):233-240.
- Zhou NB, Wang KG, Fu ZJ. Effect of morphine and a low dose of ketamine on the T cells of patients with refractory cancer pain in vitro. Oncol Lett 2019;18(4):4230-4236.
- Beilin B, Shavit Y, Hart J, Mordashov B, Cohn S, et al. Effects of anesthesia based on large versus small doses of fentanyl on natural killer cell cytotoxicity in the perioperative period. Anesth Analg 1996;82(3):492-497.
- Vujanovic NL, Basse P, Herberman RB, Whiteside TL. Antitumor functions of natural killer cells and control of metastases. Methods 1996;9(2):394-408.
- Jobin G, Rodriguez-Suarez R, Betito K. Association between natural killer cell activity and colorectal cancer in high-risk subjects undergoing colonoscopy. Gastroenterology 2017;153(4):980-987.
- Ren Q, Hua L, Zhou X, Cheng Y, Lu M, et al. Effects of a single sub-anesthetic dose of ketamine on postoperative emotional responses and inflammatory factors in colorectal cancer patients. Front Pharmacol 2022;13:818822.
- Cho JS, Kim NY, Shim JK, Jun JH, Lee S, et al. The immunomodulatory effect of ketamine in colorectal cancer surgery: A randomized-controlled trial. Can J Anaesth 2021;68(5):683-692.
- Zhou N, Fu Z, Li H, Wang K. Ketamine, as adjuvant analgesics for patients with refractory cancer pain, does affect IL-2/IFN-gamma expression of T cells in vitro?: A prospective, randomized, double-blind study. Medicine (Baltimore) 2017;96(16):e6639.
- Stollings LM, Jia LJ, Tang P, Dou H, Lu B, et al. Immune modulation by volatile anesthetics. Anesthesiology 2016;125(2):399-411.
- Cronin AJ, Aucutt-Walter NM, Budinetz T, Bonafide CP, DiVittore NA, et al. Low-dose remifentanil infusion does not impair natural killer cell function in healthy volunteers. Br J Anaesth 2003;91(6):805-809.
- Zhang J, Ma Q, Li W, Li X, Chen X. S-Ketamine attenuates inflammatory effect and modulates the immune response in patients undergoing modified radical mastectomy: A prospective randomized controlled trial. Front Pharmacol 2023;14:1128924.
- Rodriguez Arango JA, Zec T, Khalife M. Perioperative ketamine and cancer recurrence: A comprehensive review. J Clin Med 2024;13(7).
- Monteith GR, Davis FM, Roberts-Thomson SJ. Calcium channels and pumps in cancer: Changes and consequences. J Biol Chem 2012;287(38):31666-31673.
- Hu J, Duan W, Liu Y. Ketamine inhibits aerobic glycolysis in colorectal cancer cells by blocking the NMDA receptor-CaMK II-c-Myc pathway. Clin Exp Pharmacol Physiol 2020;47(5):848-856.
- He GN, Bao NR, Wang S, Xi M, Zhang TH, et al. Ketamine induces ferroptosis of liver cancer cells by targeting lncRNA PVT1/miR-214-3p/GPX4. Drug Des Devel Ther 2021;15:3965-3978.
- Duan W, Hu J, Liu Y. Ketamine inhibits colorectal cancer cells malignant potential via blockage of NMDA receptor. Exp Mol Pathol 2019;107:171-178.
- Hofbauer R, Moser D, Hammerschmidt V, Kapiotis S, Frass M. Ketamine significantly reduces the migration of leukocytes through endothelial cell monolayers. Crit Care Med 1998;26(9):1545-1549.
- Chen RM, Wu CH, Chang HC, Wu GJ, Lin YL, et al. Propofol suppresses macrophage functions and modulates mitochondrial membrane potential and cellular adenosine triphosphate synthesis. Anesthesiology 2003;98(5):1178-1185.
- Melanson B, Leri F. Effect of ketamine on the physiological responses to combined hypoglycemic and psychophysical stress. IBRO Neurosci Rep 2021;11:81-87.
- Chen LK, Shih CH, Chen SS, Huang ZX, Chang YJ, et al. Ketamine promotes breast tumor growth in a mouse breast tumor model involving with high expression of miR-27b-3p and EGFR. Invest New Drugs 2022;40(6):1165-1172.
- Malsy M, Gebhardt K, Gruber M, Wiese C, Graf B, et al. Effects of ketamine, s-ketamine, and MK 801 on proliferation, apoptosis, and necrosis in pancreatic cancer cells. BMC Anesthesiol 2015;15:111.
- Malsy M, Graf B, Bundscherer A. The effects of analgesics and local anesthetics on gene transcription mediated by NFATc2 and Sp1 in pancreatic carcinoma. Anticancer Res 2019;39(9):4721-4728.
- Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev 2003;17(18):2205-2232.
- Short B, Fong J, Galvez V, Shelker W, Loo CK. Side-effects associated with ketamine use in depression: A systematic review. Lancet Psychiatry 2018;5(1):65-78.
- Winstock AR, Mitcheson L, Gillatt DA, Cottrell AM. The prevalence and natural history of urinary symptoms among recreational ketamine users. BJU Int 2012;110(11):1762-1766.
- Chu PS, Ma WK, Wong SC, Chu RW, Cheng CH, et al. The destruction of the lower urinary tract by ketamine abuse: A new syndrome? BJU Int 2008;102(11):1616-1622.
- Cheung RY, Chan SS, Lee JH, Pang AW, Choy KW, et al. Urinary symptoms and impaired quality of life in female ketamine users: Persistence after cessation of use. Hong Kong Med J 2011;17(4):267-273.
- Noppers IM, Niesters M, Aarts L, Bauer MCR, Drewes AM, et al. Drug-induced liver injury following a repeated course of ketamine treatment for chronic pain in CRPS type 1 patients: A report of 3 cases. Pain 2011;152(9):2173-2178.
- Sear JW. Ketamine hepato-toxicity in chronic pain management: Another example of unexpected toxicity or a predicted result from previous clinical and pre-clinical data? Pain 2011;152(9):1946-1947.
- Thune A, Jivegard L, Pollard H, Moreau J, Schwartz JC, et al. Location of enkephalinase and functional effects of [Leu5] enkephalin and inhibition of enkephalinase in the feline main pancreatic and bile duct sphincters. Clin Sci (Lond) 1992;82(2):169-173.
- Lo RS, Krishnamoorthy R, Freeman JG, Austin AS. Cholestasis and biliary dilatation associated with chronic ketamine abuse: A case series. Singapore Med J 2011;52(3):e52-55.
- Morgan CJ, Rossell SL, Pepper F, Smart J, Blackburn J, et al. Semantic priming after ketamine acutely in healthy volunteers and following chronic self-administration in substance users. Biol Psychiatry 2006;59(3):265-272.
- Hartvig P, Valtysson J, Lindner KJ, Kristensen J, Karlsten R, et al. Central nervous system effects of subdissociative doses of (S)-ketamine are related to plasma and brain concentrations measured with positron emission tomography in healthy volunteers. Clin Pharmacol Ther 1995;58(2):165-173.
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 1994;51(3):199-214.
- Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, et al. NMDA receptor function and human cognition: The effects of ketamine in healthy volunteers. Neuropsychopharmacology 1996;14(5):301-307.
- Narendran R, Frankle WG, Keefe R, Gil R, Martinez D, et al. Altered prefrontal dopaminergic function in chronic recreational ketamine users. Am J Psychiatry 2005;162(12):2352-2359.
- Koffler SP, Hampstead BM, Irani F, Tinker J, Kiefer RT, et al. The neurocognitive effects of 5 day anesthetic ketamine for the treatment of refractory complex regional pain syndrome. Arch Clin Neuropsychol 2007;22(6):719-729.
- Murrough JW, Burdick KE, Levitch CF, Perez AM, Brallier JW, et al. Neurocognitive effects of ketamine and association with antidepressant response in individuals with treatment-resistant depression: A randomized controlled trial. Neuropsychopharmacology 2015;40(5):1084-1090.
- Murrough JW, Wan LB, Iacoviello B, Collins KA, Solon C, et al. Neurocognitive effects of ketamine in treatment-resistant major depression: association with antidepressant response. Psychopharmacology (Berl) 2013.
- WHO model prescribing information: drugs used in anesthesia. 1989. Jh2929e/4.html (accessed Feb 1, 2017).
- Liu JJW, Ein N, Gervasio J, Baker C, Plouffe R, et al. Ketamine in the effective management of chronic pain, depression, and posttraumatic stress disorder for Veterans: A meta-analysis and systematic review. Front Psychiatry 2024;15:1338581.
- Moreton JE, Meisch RA, Stark L, Thompson T. Ketamine self-administration by the rhesus monkey. J Pharmacol Exp Ther 1977;203(2):303-309.
- Blachut M, Solowiow K, Janus A, Ruman J, Cekus A, et al. [A case of ketamine dependence]. Psychiatr Pol 2009;43(5):593-599.
- Muller A, Lemos D. Cancer pain: Beneficial effect of ketamine addition to spinal administration of morphine-clonidine-lidocaine mixture. Ann Fr Anesth Reanim 1996, 15(3):271-276.
- Stubhaug A, Breivik H, Eide PK, Kreunen M, Foss A. Mapping of punctuate hyperalgesia around a surgical incision demonstrates that ketamine is a powerful suppressor of central sensitization to pain following surgery. Acta Anaesthesiol Scand 1997, 41(9):1124-1132.
- Galili SF, Bech BH, Kirkegaard H, Ahrensberg J, Nikolajsen L. Low-dose ketamine as an adjunct to morphine: A randomized controlled trial among patients with and without current opioid use. Acad Emerg Med 2024.
- Mischel RA, Muchhala KH, Dewey WL, Akbarali HI. The "culture" of pain control: A review of opioid-induced dysbiosis (OID) in antinociceptive tolerance. J Pain 2020, 21(7-8):751-762.
- Varrassi G, Fusco M, Skaper SD, Battelli D, Zis P, et al. A pharmacological rationale to reduce the incidence of opioid induced tolerance and hyperalgesia: A review. Pain Ther 2018, 7(1):59-75.
- Colvin LA, Bull F, Hales TG. Perioperative opioid analgesia-when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet 2019, 393(10180):1558-1568.
- Martyn JAJ, Mao J, Bittner EA. Opioid tolerance in critical illness. Reply. N Engl J Med 2019, 380(16):e26.
- Rodriguez-Munoz M, Sanchez-Blazquez P, Vicente-Sanchez A, Berrocoso E, Garzon J. The mu-opioid receptor and the NMDA receptor associate in PAG neurons: implications in pain control. Neuropsychopharmacology 2012, 37(2):338-349.
- Lovick TA. Integrated activity of cardiovascular and pain regulatory systems: Role in adaptive behavioural responses. Prog Neurobiol 1993, 40(5):631-644.
- Fusumada K, Yokoyama T, Miki T, Wang ZY, Yang W, et al. c-Fos expression in the periaqueductal gray is induced by electroacupuncture in the rat, with possible reference to GABAergic neurons. Okajimas Folia Anat Jpn 2007, 84(1):1-9.
- Ho YC, Lee HJ, Tung LW, Liao YY, Fu SY, et al. Activation of orexin 1 receptors in the periaqueductal gray of male rats leads to antinociception via retrograde endocannabinoid (2-arachidonoylglycerol)-induced disinhibition. J Neurosci 2011, 31(41):14600-14610.
- Yaksh TL, Al-Rodhan NR, Jensen TS. Sites of action of opiates in production of analgesia. Prog Brain Res 1988, 77:371-394.
- Sanchez-Blazquez P, Rodriguez-Munoz M, Berrocoso E, Garzon J. The plasticity of the association between mu-opioid receptor and glutamate ionotropic receptor N in opioid analgesic tolerance and neuropathic pain. Eur J Pharmacol 2013, 716(1-3):94-105.
- Su YL, Huang J, Wang N, Wang JY, Luo F. The effects of morphine on basal neuronal activities in the lateral and medial pain pathways. Neurosci Lett 2012, 525(2):173-178.
- Ding J, Yu YM, Luo M, Fang X, Tan DD, et al. Thrifty effect of subanesthetic-dose S-ketamine on postoperative opioids and its safety and analgesic effectiveness: A prospective, triple-blind, randomized controlled, polycentric clinical trial. Ibrain 2023, 9(2):171-182.
- Subramaniam K, Subramaniam B, Steinbrook RA. Ketamine as adjuvant analgesic to opioids: A quantitative and qualitative systematic review. Anesth Analg 2004, 99(2):482-495, table of contents.
- Fuller RG, Kikla EM, Fawcett APW, Hesling JD, Keenan S, et al. Low-dose ketamine for acute pain: A narrative review. Am J Emerg Med 2024, 86:41-55.
- Jabbour HJ, Naccache NM, Jawish RJ, Abou Zeid HA, Jabbour KB, et al. Ketamine and magnesium association reduces morphine consumption after scoliosis surgery: prospective randomised double-blind study. Acta Anaesthesiol Scand 2014, 58(5):572-579.
- Guo J, Zhao F, Bian J, Hu Y, Tan J. Low-dose ketamine versus morphine in the treatment of acute pain in the emergency department: A meta-analysis of 15 randomized controlled trials. Am J Emerg Med 2024, 76:140-149.
- Lauretti GR, Gomes JM, Reis MP, Pereira NL. Low doses of epidural ketamine or neostigmine, but not midazolam, improve morphine analgesia in epidural terminal cancer pain therapy. J Clin Anesth 1999, 11(8):663-668.
- Sen S, Aydin ON, Aydin K. Beneficial effect of low-dose ketamine addition to epidural administration of morphine-bupivacaine mixture for cancer pain in two cases. Pain Med 2006, 7(2):166-169.
- Park K, Kim JJ, Oh SB, Oh SY, Hong YJ, et al. A phase ii study about efficacy and safety of the continuous intravenous infusion of ketamine as adjuvant to opioids in terminally ill cancer patients with refractory cancer pain (CIVIK Trial). Am J Hosp Palliat Care 2024:10499091241252977.
- Singh V, Gillespie TW, Lane O, Spektor B, Zarrabi AJ, et al. A dose-escalation clinical trial of intranasal ketamine for uncontrolled cancer-related pain. Pharmacotherapy 2022, 42(4):298-310.
- Kozek SA, Sator-Katzenschlager S, Kress HG. Intrathecal S(+)-ketamine in refractory neuropathic cancer pain. Pain 2006, 121(3):283-284.
- Vincent JP, Cavey D, Kamenka JM, Geneste P, Lazdunski M. Interaction of phencyclidines with the muscarinic and opiate receptors in the central nervous system. Brain Res 1978, 152(1):176-182.
- Ben-Azu B, Fokoua AR, Annafi OS, Adebayo OG, Del Re EC, et al. Effective action of silymarin against ketamine-induced schizophrenia in male mice: Insight into the biochemical and molecular mechanisms of action. J Psychiatr Res 2024, 179:141-155.
- Wang T, Zheng Q, Yang Q, Guo F, Cui H, et al. The metabolic clock of ketamine abuse in rats by a machine learning model. Sci Rep 2024, 14(1):18867.
- Jiang Y, Li T, Qian Y, Zuo X, Liu J. Morphine in combination with ketamine improves cervical cancer pain and suppresses immune function via the JAK3/STAT5 pathway. Pain Res Manag 2022, 2022:9364365.
- Xiang J, Cao C, Chen J, Kong F, Nian S, et al. Efficacy and safety of ketamine as an adjuvant to regional anesthesia: A systematic review and meta-analysis of randomized controlled trials. J Clin Anesth 2024, 94:111415.
- Hardy J, Quinn S, Fazekas B, Plummer J, Eckermann S, et al. Randomized, double-blind, placebo-controlled study to assess the efficacy and toxicity of subcutaneous ketamine in the management of cancer pain. J Clin Oncol 2012, 30(29):3611-3617.
- Yang CY, Wong CS, Chang JY, Ho ST. Intrathecal ketamine reduces morphine requirements in patients with terminal cancer pain. Can J Anaesth 1996, 43(4):379-383.
- Mercadante S, Arcuri E, Tirelli W, Casuccio A. Analgesic effect of intravenous ketamine in cancer patients on morphine therapy: A randomized, controlled, double-blind, crossover, double-dose study. J Pain Symptom Manage 2000, 20(4):246-252.
- Rodriguez-Mayoral O, Perez-Esparza R, Dominguez-Ocadio G, Allende-Perez S. Ketamine as augmentation for the treatment of major depression and suicidal risk in advanced cancer: Case report. Palliat Support Care 2020, 18(1):110-112.
- Sexton J, Atayee RS, Bruner HC. Case report: Ketamine for pain and depression in advanced cancer. J Palliat Med 2018, 21(11):1670-1673.
- Zanicotti CG, Perez D, Glue P. Mood and pain responses to repeat dose intramuscular ketamine in a depressed patient with advanced cancer. J Palliat Med 2012, 15(4):400-403.
- Stefanczyk-Sapieha L, Oneschuk D, Demas M. Intravenous ketamine "burst" for refractory depression in a patient with advanced cancer. J Palliat Med 2008, 11(9):1268-1271.
Table of Contents
- Abstract
- Keywords
- Introduction
- Review of Research on the Comorbidities of Depression and Cancer
- Research on Cancer Pain and Depression
- Research on Ketamine Reducing Cancer Pain and Depression
- Ketamine and Tumor Recurrence
- Diagnosis and Treatment Status of Adverse Effects of Ketamine
- Research on Combination Use of Ketamine and Opioids
- Research Progress in the Discovery of Ketamine in Patients with CPD
- Conclusion
- Funding
- Conflict of Interests Statement
- Figure 1
- Figure 2
- References