Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/061
RESEARCH ARTICLE OPEN ACCESS
Gene therapy with HSV encoding p55TNFR gene for HIV neuropathic pain: an evidence-based mini-review
Hirotsugu Kanda1,2, Shue Liu1, Megumi Kanao1,2, Hyun Yi1, Takafumi Iida1,2, Wan Huang1, Takayuki Kunisawa2, David A. Lubarsky1 and Shuanglin Hao1
1Department of Anesthesiology, University of Miami Miller School of Medicine, Miami, Florida 33136
2Department of Anesthesiology and Critical Care Medicine, Asahikawa Medical University, Japan 078-8510
#The work was partially reported at the 2016 annual meeting of American Society of Anesthesiologists in Chicago.
Shuanglin Hao, MD, PhD, Professor & Director for Preclinical and Basic Research, Department of Anesthesiology, University of Miami Miller School of Medicine Miami, FL33136, USA, Email: shao@med.miami.edu
Editor: Yuan-Xiang Tao, Ph.D., M.D., Professor and Vice Chair, Director, Center for Pain Medicine Research Department of Anesthesiology, Editor in Chief, The Translational Perioperative and Pain Medicine, Rutgers, The State University of New Jersey, New Jersey Medical School, 185 S. Orange Ave., MSB, E-661 Newark, NJ 07103. Phone: 973-972-9812 (O), Email: yt211@njms.rutgers.edu
Received: August 12, 2017 | Accepted: September 21, 2017 | Published: October 03, 2017
Citation: Kanda H, Liu S, Kanao M, Yi H, Iida T, et al. Gene therapy with HSV encoding p55TNFR gene for HIV neuropathic pain: an evidence-based minireview. Transl Perioper & Pain Med 2017; 4 (4):24-32.
Abstract
While effective antiretroviral treatment makes human immunodeficiency virus (HIV)-related death decreased dramatically, neuropathic pain becomes one of the most common complications in patients with HIV/acquired immunodeficiency syndrome (AIDS). The exact mechanisms of HIV-related neuropathic pain are not well understood yet, and no effective therapy is for HIV-pain. Evidence has shown that proinflammatory factors (e.g., tumor necrosis factor alpha (TNFα)) released from glia, are critical to contributing to chronic pain. Preclinical studies have demonstrated that non-replicating herpes simplex virus (HSV)-based vector expressing human enkephalin reduces inflammatory pain, neuropathic pain, or cancer pain in animal models. In this review, we describe recent advances in the use of HSV-based gene transfer for the treatment of HIV pain, with a special focus on the use of HSV-mediated soluble TNF receptor I (neutralizing TNFα in function) in HIV neuropathic pain model.
Keywords
HIV, Neuropathic pain, Soluble TNF receptor, and Gene therapy
Introduction
The United States Centers for Disease Control reports that an estimated 1.1 million people were living with human immunodeficiency virus (HIV) at the end of 2014 and that 39,513 people in 2015 were diagnosed with HIV infection in the United States (https://www.cdc.gov/hiv/basics/statistics.html, last date accessed June 24, 2017). Although effective antiretroviral therapy (ART) makes HIV become a treatable, chronic disease [1,2], new challenges are emerging in managing HIV. Chronic pain becomes one of the most common complications in patients with HIV/acquired immunodeficiency syndrome (AIDS). HIV-related pain is often underestimated in HIV/AIDS patients while the main focus is on immunosuppression and opportunistic infections. HIV neuropathic pain (HIV-NP) is refractory, and the current available chronic pain therapies are not effective to HIV-NP. This article reviews current researches from our work and others focusing on the pathophysiological mechanisms of HIV-neuropathic pain and gene therapy.
HIV-Related Neuropathic Pain
HIV sensory neuropathies contain distal sensory polyneuropathy as results of both HIV infection and antiretroviral drug-induced toxic neuropathies [3-6]. Clinical characteristics of distal sensory polyneuropathy and ART-induced toxic neuropathies are very similar. Neurotoxic ART has even been removed from pharmacies entirely in developed countries. Evidence shows that many people with HIV alive today, have ever been on numerous therapeutic regimens with neurotoxic drugs, and that they have already developed persistent painful neuropathy [7,8]. HIV-NP is typically bilateral, of gradual onset, and described as 'aching', 'painful numbness', or 'burning' [9]. Pathological feature of HIV-NP includes loss of sensory neurons of the dorsal root ganglion (DRG), Wallerian degeneration of the long axons in distal area, infiltration of macrophage into the DRG, and a 'dying back' sensory neuropathy [10-14]. However, the precise mechanisms of HIV-NP remain unknown yet and no effective therapy for HIV-NP.
Proinflammatory factors
Early studies have demonstrated that glia infected/activated by HIV release proinflammatory factors, such as tumor necrosis factor alpha (TNFα) and interleukin 1 (IL-1) [15]. Infiltration of inflammatory lymphocyte and macrophage to the DRG of AIDS patients produces pro-inflammatory cytokines including TNFα [12,16-20]. There is an increased TNFα in human CSF [21-25] and brain tissue [25-28] in patients with HIV. An interaction of TNFα and HIV infection enhances toxic chemokine products [29,30]. It is known that proinflammatory cytokines play an important role in the development and maintenance of neuropathic pain [31-35]. Proinflammatory mediators are critical to enhancing HIV-NP [36]. Intrathecal administration of gp120 induces acute pain and spinal proinflammatory cytokine release [37]. Peripheral gp120 increases TNFα within the nerve trunk [38], intense glial activation in the spinal cord in parallel with neuropathic pain behaviors [38]. We have reported that peripheral gp120 application onto the rat sciatic nerve upregulates TNFα in the L4/5 DRGs and spinal cord [39]. Systemic 2',3'-dideoxycytidine (ddC), one drug of ART lowers mechanical threshold [40,41] and increases both mRNA and protein of TNFα in the spinal cord dorsal horn (SCDH) [41]. Inhibition of TNFα or soluble TNF receptor reduces mechanical allodynia induced by gp120 application [41].
Therefore, it is possible that TNFα signal is involved in the induction and/or progression of HIV-NP.
Reactive oxygen species and C/EBPβ in HIV
Oxidative stress evokes many signaling events [42]. Mitochondria are the main source of reactive oxygen species (ROS). ROS plays a role in different pain models [43-48]. ROS scavengers produce a strong antinociceptive effect in persistent pain models [49]. Oxidative stress is involved in the pathogenesis of neuroAIDS [50]. HIV infection and ART can evoke rapid neurotoxicity [51]. Either HIV gp120 or ddC plays a role in initiation and/or intensification of ROS [52,53]. Intrathecal gp120 induces spinal release of nitric oxide (NO) as well as proinflammatory cytokines; pretreatment with NO synthase (NOS) inhibitor abolishes gp120-induced mechanical allodynia [54]. Importantly, ROS evoked by HIV infection, induces apoptosis through TNFα and its receptors [52]. Mitochondrial DNA (mtDNA) is critical for oxidative phosphorylation complex I proteins. DNA poly-merase-γ is important for replication of mtDNA. ARTs inhibit Poly-merase-γ, resulting in mitochondrial respiratory chain dysfunction and oxidative phosphorylation deficits [51]. Systemic ddC induces neuropathic pain and lowers the activity of endogenous manganese superoxide dismutase (SOD2) in the SCDH; ROS scavengers significantly reduce mechanical allodynia [55].
CCAAT/enhancer binding proteins (C/EBPs) are transcriptional factors in cell development and induction of inflammatory factors in the peripheral and central nervous system [56]. C/EBPβ plays an important role in a variety of HIV disease stages [57]. An increase in C/EBPβ mRNA is found in the brain tissue of HIV-1 encephalitis patients [58]. We have found that combination of peripheral gp120 with systemic ddC increases pC/EBPβ in the SCDH [59], suggesting that pC/EBPβ plays a role in HIV-NP.
HSV Vector for Gene Therapy of Neuropathic Pain
During natural infection of herpes simplex virus (HSV), HSV is carried by retrograde axonal transport from the site of original inoculation to the neuronal perikaryon. Latently infected neurons function normally and are not rejected by the host immune response (see review [60,61]). HSV-1 genome is a linear double-stranded DNA, and has more than 75 genes coded in the 152 kb genome [60,61]. HSV genes are expressed in a well-ordered temporal cascade of immediate early (IE) genes, followed by early genes, and subsequently late gene products; both early genes and the late genes require synthesis of IE gene products [60,61]. Deleting essential IE genes from the HSV genome makes it non-replicating recombinant [62], but the virus are still able to be used to effectively deliver target gene products [63,64]. Gene transfer mediated by HSV vector may provide a promising approach to the management of neuropathic pain. HSV vector encoding human preproenkephalin gene after transduction of DRG neurons by hindpaw injection [65], produces an antinociceptive effect in different pain models [66-68]. We have reported that HSV vectors expressing enkephalin, p55 TNF soluble receptor (p55TNFSR), interleukin-10, and interleukin-4 produce antinociceptive effects in preclinical pain models [69-75]. Fink and colleagues reported phase 1 clinical trial using HSV vector encoding human preproenkephalin in patients with cancer pain [76]. The clinical trial assessed the safety and explored the potential efficacy of this approach in humans, indicating that it may be effective in reducing cancer pain [77].
The distribution of systemically administered drugs to the brain may be limited by the blood-brain barrier [65], and they produce systemic side effects. Gene transfer that permanently release gene products, might be a useful alternative to regular pharmacological approaches [65]. Gene transfer of HSV vector may represent a platform technology---nerve targeting drug delivery system [77]. Viral vectors, however, show toxicity and inflammation from 'leaky' expression of viral genes and reaction to the vector coat protein in pre-immune animals [65,78]. Despite these limitations, ours and other studies have shown that HSV vector is still a highly effective gene delivery approach to treating peripheral and central nervous diseases [65,79,80].
TNFSR mediated by HSV vector produces antiallodynic effect in HIV-NP
Our report has shown that the HIV gp120 application onto the sciatic nerve induces upregulation of TNFα, C-X-C chemokine receptor type 4 (CXCR4, a co-receptor of HIV), stromal cell-derived factor 1-α (SDF1-α, CXCR4 ligand) in both the DRG and the lumbar spinal dorsal horn [81]. Soluble TNF receptor (TNFSR) blocks bioactivity of TNFα. HSV vector encoding p55TNFSR gene (T0TNFSR) reduces mechanical allodynia and lowers TNFα, CXCR4 and SDF1-α induced by gp120 in the DRG and SCDH [81], suggesting that the pathway of TNFα to the CXCR4/SDF1 has an important role in the HIV-NP and that inhibiting proinflammatory cytokines/chemokines reduce neuropathic pain. In another model of HIV-NP induced by intraperitoneal ddC [40], ddC induces upregulation of TNFα, SDF1-α, and CXCR4 in both the lumbar spinal cord and the L4/5 DRG; T0TNFSR reduced mechanical allodynia and suppressed TNFα, SDF1-α, and CXCR4 in the lumbar SCDH and DRG [82], indicating that TNFα is involved in the ARTs-related pain through the SDF1-α/CXCR4 system.
We have reported that combination of peripheral gp120 with systemic ddC (gp120/ddC) lowers mechanical threshold for more than 3 weeks, and that the minimum of mechanical threshold occurs around 2 weeks after gp120/ddC [59,83,84]. Previous studies show that HSV vector T0TNFSR reduces neuropathic pain induced by spinal nerve injury [69]. In gp120/ddC model, 2 weeks post HSV vector, T0TNFSR significantly reduced foot withdrawal frequencies (Figure 1), and increased the expression of soluble TNFRI in the L4/5 DRG (Figure 2).
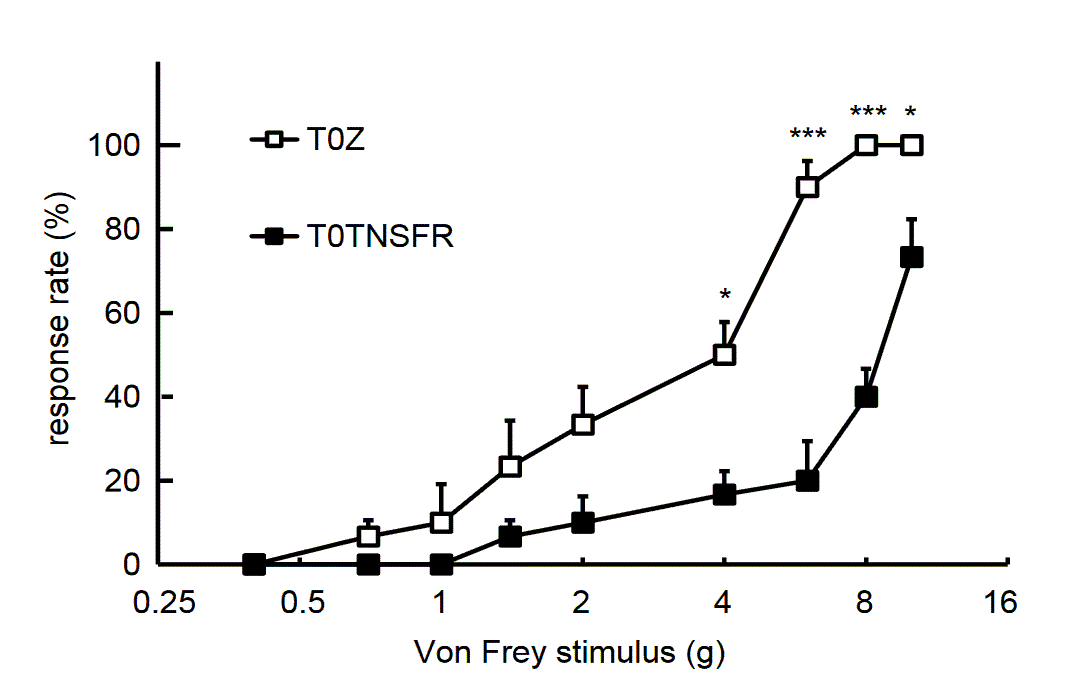
Figure 1: Antinociceptive effect of p55TNFRI mediated by HSV vectors. Mechanical sensitivity was examined through the measurement of foot withdrawal frequencies to a sequential series of calibrated von Frey filaments applied in ascending order to the plantar surface of the foot (98). HSV vector T0TNFSR or T0Z was inoculated into the hindpaws 1 week post gp120/ddC. The occurrence of foot withdrawal for each trial was expressed as a percentage response frequency. Two weeks after HSV vectors, foot withdrawal frequencies to calibrated von Frey filaments in rats with subcutaneous inoculation of T0TNFSR were significantly lower than that in T0Z at filaments of 3.6, 5.5, 8.5, and 11.8 gram, * P< 0.05, ***P< 0.001 vs. T0TNFSR, t test, n=6.
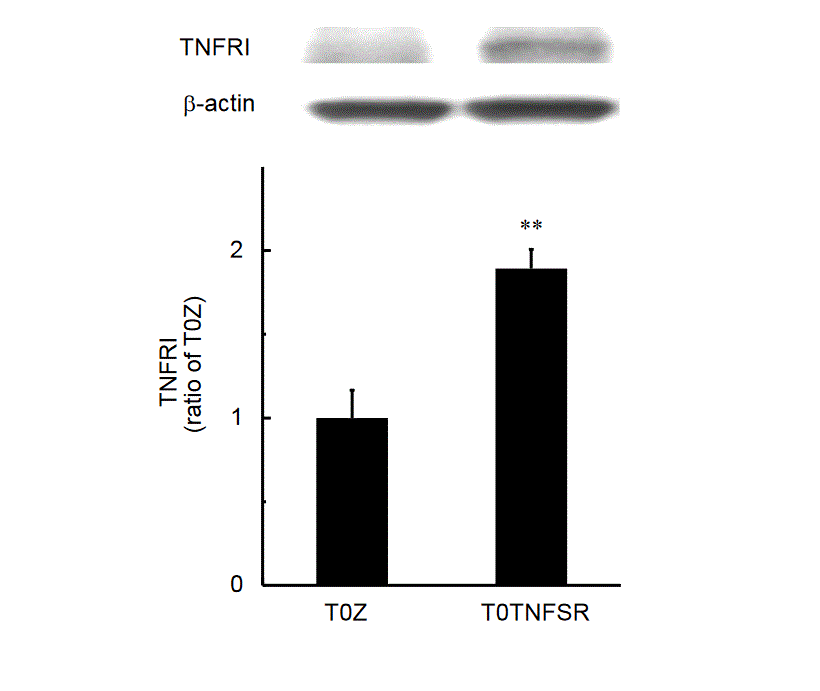
Figure 2: The expression of p55TNFSR mediated by the HSV vectors. One week after gp120/ddC, T0TNFSR or T0Z was inoculated into the hindpaws. On day 14 post HSV vector, the L4/5 dorsal root ganglion (DRG) was harvested, and western blot assays were conducted for testing TNFRI. T0TNFSR injection significantly induced the expression of TNFRI compared with T0Z in the L4/5 DRG, **P < 0.01 vs. T0Z, t test, n= 6.
TNFSR mediated by the HSV vector reduces mitochondrial superoxide in gp120/ddC model
Oxidative stress causes many signaling events [42]. HIV gp120 or ddC induces ROS [52,53]. HIV gp120 application onto the sciatic nerve upregulates spinal mitochondrial superoxide [73,85]. We reported that gp120/ddC increased spinal mitochondrial superoxide [59,84] using MitoSox positive cell imaging (a marker of mitochondrial superoxide) [86,87]. Figure 3A-C showed the representative MitoSox positive cell imaging in the gp120/ddC model. The increased number of MitoSox positive cells in the gp120/ddC model was decreased by HSV vector T0TNFSR (Figure 3D), suggesting that TNFSR suppresses neuropathic pain through reducing spinal ROS.
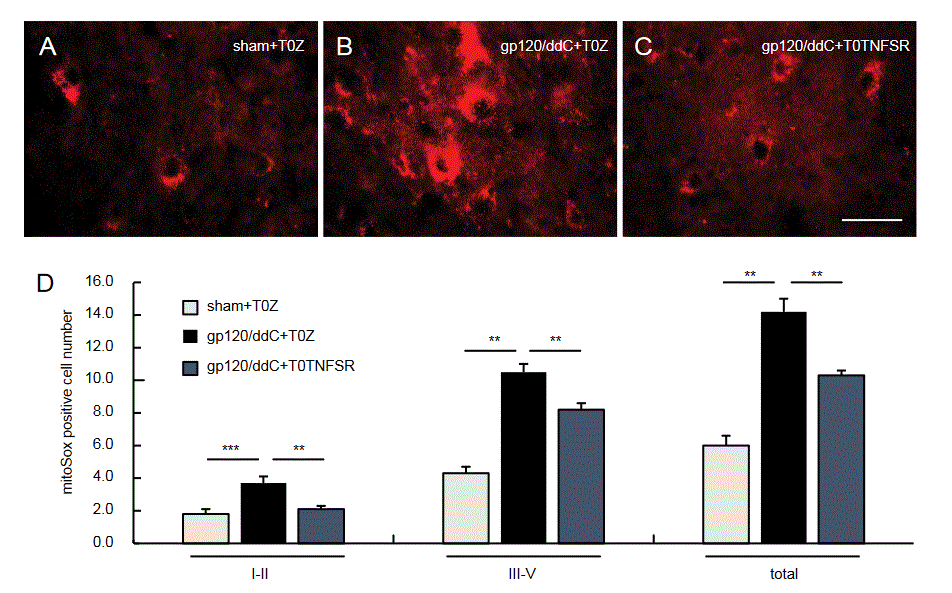
Figure 3: The effect of p55TNFSR mediated by the HSV vectors on mitochondrial superoxide in the SCDH at 2 weeks post HSV vectors. One week post gp120/ddC, neuropathic rats received hindpaw injection of T0TNFSR or T0Z into ipsilateral hindpaw of rats. Two weeks after HSV vector, MitoSox Red was intrathecally injected 70 min prior to perfusion. The representative image of MitoSox red for mitochondrial superoxide in sham+T0Z, gp120/ddC+T0Z, and gp120/ddC+T0TNFSR, was shown in Figure A, B, and C, respectively, scale bar, 50µm. (D) The number of mitochondrial superoxide positive cells in the SCDH lamina I-II and III-V was shown, **P < 0.01, ***P < 0.001, one way ANOVA with post hoc PLSD test, mean ± SEM, n=5-6 rats.
TNFSR mediated by the HSV vector inhibits pC/EBPβ in the gp120/ddC neuropathic pain model
C/EBP plays an role in induction of inflammatory mediators in CNS [56]. HIV patients show upregulation of C/EBPβ mRNA in the brain tissue [58]. We have shown that HIV-NP increases phosphorylation of C/EBPβ (pC/EBPβ) [59]. Figure 4A-C revealed the representative pC/EBPβ-IR images in the gp120/ddC model. Treatment with gp120/ddC increased pC/EBPβ-IR expression; the upregulated pC/EBPβ-IR was suppressed by T0TNFSR (Figure 4D), suggesting that TNFSR reduces neuropathic pain through decreasing spinal pC/EBPβ.
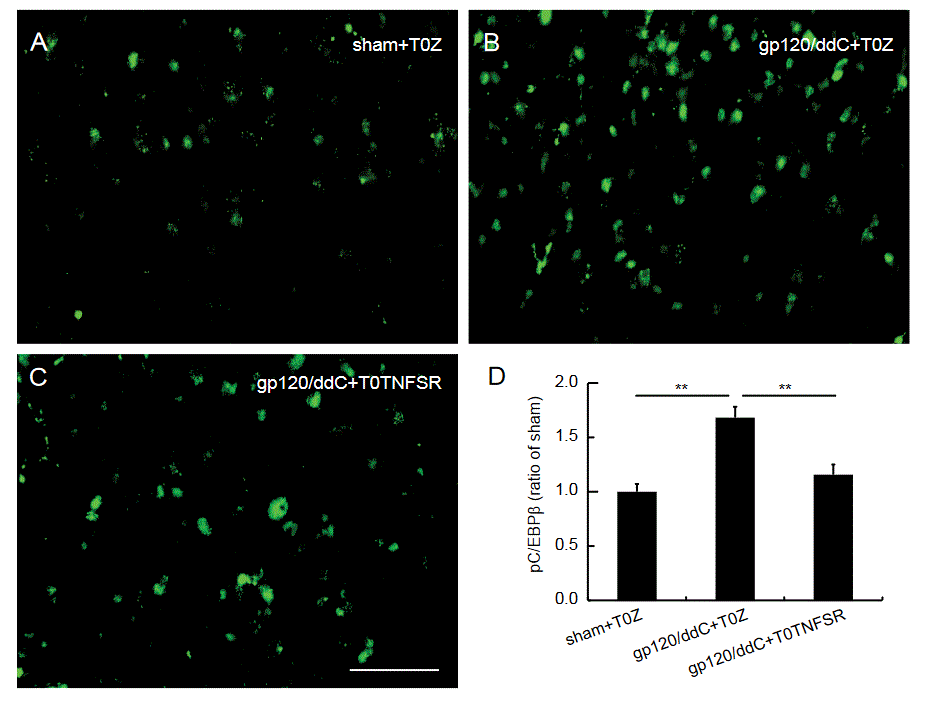
Figure 4: The effect of p55TNFSR mediated by the HSV vectors on pC/EBPβ in the SDH at 2 weeks post HSV vectors. One week post gp120/ddC, neuropathic rats received hindpaw injection of T0TNFSR or T0Z into ipsilateral hindpaw of rats. Two weeks after HSV vector, spinal pC/EBPβ-IR was examined using immunohistochemistry. The representative images of pC/EBPβ-IR in sham+T0Z, gp120/ddC+T0Z, and gp120/ddC+T0Z were shown in Figure A, B, and C, respectively, scale bar, 50µm. (D) The quantitative signals of pC/EBPβ-IR in the SCDH were shown. There was an increase in pC/EBPβ-IR in the group of gp120/ddC+T0Z compared to sham+T0Z (P=0.01); the expression of pCREB-IR in gp120/ddC+T0TNFSR was lower than that in gp120/ddC+T0TNFSR (P = 0.01), one way ANOVA with post hoc PLSD test, mean ± SEM, n=6 rats.
The relationship of TNFα/TNF receptor activity and ROS or C/EBPβ in HIV-NP is still not clear. HIV gp120/ddC induces release of TNFα [83]. Through TNF receptor, TNFα triggers a cascade of events [88]. TNFα activates NMDA receptors to increases Ca2+ influx [89]. Indeed, HIV gp120 increases intracellular free Ca2+ concentration in the mice SCDH cells [90]. ddC also increases spinal cytosolic Ca2+ concentration in painful neuropathy [91]. There is an interplay between cytosolic Ca2+ and mitochondrial ROS [92-94]. Spinal pCREB may make a contribution to the development of chronic pain [95]. Cytosolic Ca2+ may induce transcriptional factor CREB regulating C/EBPβ activity [96]. CREB binds C/EBPβ gene promoter, inducing the endogenous C/EBPβ expression [97]. Therefore, it is possible that TNFα-TNFR induces ROS, or pCREB/ C/EBPβ in HIV-NP, which need to be examined in the near future.
In summary, glia infected or activated after HIV release proinflammatory factors, such as TNFα. TNFα-TNF receptor signal may induce ROS or C/EBPβ in HIV-NP through complex pathway in the model of HIV-NP. Gene transfer using the HSV vector encoding the gene of TNF soluble receptor reduced neuropathic pain in animal studies, providing additional potential approach for successful treatment of HIV neuropathic pain.
Conflict of Interest
All authors declare no conflict of interest in the work.
Acknowledgements
Supported by grants from the National Institutes of Health DA026734 (SH), DA025527 (SH), NS066792 (SH) and DA34749 (SH), and by the Department of Anesthesiology, the University of Miami Miller School of Medicine, Miami, FL.
References
- Colvin CJ. HIV/AIDS, chronic diseases and globalisation. Globalization and health 2011;7:31.
- Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013;382:1525-33.
- Keswani SC, Pardo CA, Cherry CL, Hoke A, McArthur JC. HIV-associated sensory neuropathies. Aids 2002;16:2105-17.
- Nath A. Neurologic Complications of Human Immunodeficiency Virus Infection. Continuum (Minneap Minn) 2015;21:1557-76.
- Hoke A, Cornblath DR. Peripheral neuropathies in human immunodeficiency virus infection. Suppl Clin Neurophysiol 2004;57:195-210.
- Cherry CL, Wadley AL, Kamerman PR. Painful HIV-associated sensory neuropathy. Pain Manag 2012;2:543-52.
- Kranick SM, Nath A. Neurologic complications of HIV-1 infection and its treatment in the era of antiretroviral therapy. Continuum (Minneap Minn) 2012;18:1319-37.
- Leger PD, Johnson DH, Robbins GK, Shafer RW, Clifford DB, Li J, McLaren PJ, Haas DW. Genome-wide association study of peripheral neuropathy with D-drug-containing regimens in AIDS Clinical Trials Group protocol 384. J Neurovirol 2014;20:304-8.
- Cornblath DR, McArthur JC. Predominantly sensory neuropathy in patients with AIDS and AIDS-related complex. Neurology 1988;38:794-6.
- Wulff EA, Wang AK, Simpson DM. HIV-associated peripheral neuropathy: epidemiology, pathophysiology and treatment. Drugs 2000;59:1251-60.
- Keswani SC, Polley M, Pardo CA, Griffin JW, McArthur JC, Hoke A. Schwann cell chemokine receptors mediate HIV-1 gp120 toxicity to sensory neurons. Ann Neurol 2003;54:287-96.
- Pardo CA, McArthur JC, Griffin JW. HIV neuropathy: insights in the pathology of HIV peripheral nerve disease. J Peripher Nerv Syst 2001;6:21-7.
- McCarthy BG, Hsieh ST, Stocks A, Hauer P, Macko C, Cornblath DR, Griffin JW, McArthur JC. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology 1995;45:1848-55.
- Verma A. Epidemiology and clinical features of HIV-1 associated neuropathies. J Peripher Nerv Syst 2001;6:8-13.
- Merrill JE, Chen IS. HIV-1, macrophages, glial cells, and cytokines in AIDS nervous system disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 1991;5:2391-7.
- Nagano I, Shapshak P, Yoshioka M, Xin K, Nakamura S, Bradley WG. Increased NADPH-diaphorase reactivity and cytokine expression in dorsal root ganglia in acquired immunodeficiency syndrome. Journal of the neurological sciences 1996;136:117-28.
- Rizzuto N, Cavallaro T, Monaco S, Morbin M, Bonetti B, Ferrari S, Galiazzo-Rizzuto S, Zanette G, Bertolasi L. Role of HIV in the pathogenesis of distal symmetrical peripheral neuropathy. Acta Neuropathol 1995;90:244-50.
- Shapshak P, Nagano I, Xin K, Bradley W, McCoy CB, Sun NC, Stewart RV, Yoshioka M, Petito C, Goodkin K, et al. HIV-1 heterogeneity and cytokines. Neuropathogenesis. Advances in experimental medicine and biology 1995;373:225-38.
- Yoshioka M, Shapshak P, Srivastava AK, Stewart RV, Nelson SJ, Bradley WG, Berger JR, Rhodes RH, Sun NC, Nakamura S. Expression of HIV-1 and interleukin-6 in lumbosacral dorsal root ganglia of patients with AIDS. Neurology 1994;44:1120-30.
- Wesselingh SL, Glass J, McArthur JC, Griffin JW, Griffin DE. Cytokine dysregulation in HIV-associated neurological disease. Adv Neuroimmunol 1994;4:199-206.
- Grimaldi LM, Martino GV, Franciotta DM, Brustia R, Castagna A, Pristera R, Lazzarin A. Elevated alpha-tumor necrosis factor levels in spinal fluid from HIV-1-infected patients with central nervous system involvement. Ann Neurol 1991;29:21-5.
- Mastroianni CM, Paoletti F, Valenti C, Vullo V, Jirillo E, Delia S. Tumour necrosis factor (TNF-alpha) and neurological disorders in HIV infection. J Neurol Neurosurg Psychiatry 1992;55:219-21.
- Perrella O, Carrieri PB, Guarnaccia D, Soscia M. Cerebrospinal fluid cytokines in AIDS dementia complex. J Neurol 1992;239:387-8.
- Ciardi M, Sharief MK, Thompson EJ, Salotti A, Vullo V, Sorice F, Cirelli A. High cerebrospinal fluid and serum levels of tumor necrosis factor-alpha in asymptomatic HIV-1 seropositive individuals. Correlation with interleukin-2 and soluble IL-2 receptor. Journal of the neurological sciences 1994;125:175-9.
- Tyor WR, Glass JD, Griffin JW, Becker PS, McArthur JC, Bezman L, Griffin DE. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol 1992;31:349-60.
- Achim CL, Heyes MP, Wiley CA. Quantitation of human immunodeficiency virus, immune activation factors, and quinolinic acid in AIDS brains. J Clin Invest 1993;91:2769-75.
- An SF, Ciardi A, Giometto B, Scaravilli T, Gray F, Scaravilli F. Investigation on the expression of major histocompatibility complex class II and cytokines and detection of HIV-1 DNA within brains of asymptomatic and symptomatic HIV-1-positive patients. Acta Neuropathol 1996;91:494-503.
- Vitkovic L, da Cunha A, Tyor WR. Cytokine expression and pathogenesis in AIDS brain. Res Publ Assoc Res Nerv Ment Dis 1994;72:203-22.
- Williams R, Dhillon NK, Hegde ST, Yao H, Peng F, Callen S, Chebloune Y, Davis RL, Buch SJ. Proinflammatory cytokines and HIV-1 synergistically enhance CXCL10 expression in human astrocytes. Glia 2009;57:734-43.
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 2001;410:988-94.
- Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. The Journal of neuroscience : the official journal of the Society for Neuroscience 2002;22:9980-9.
- Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends in neurosciences 2001;24:450-5.
- DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist 2004;10:40-52.
- Leung L, Cahill CM. TNF-alpha and neuropathic pain--a review. J Neuroinflammation 2010;7:27.
- Gao YJ, Zhang L, Ji RR. Spinal injection of TNF-alpha-activated astrocytes produces persistent pain symptom mechanical allodynia by releasing monocyte chemoattractant protein-1. Glia 2010;58:1871-80.
- Hao S. The Molecular and Pharmacological Mechanisms of HIV-Related Neuropathic Pain. Current Neuropharmacology 2013;11:499-512.
- Milligan ED, O'Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, Watkins LR. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. The Journal of neuroscience : the official journal of the Society for Neuroscience 2001;21:2808-19.
- Herzberg U, Sagen J. Peripheral nerve exposure to HIV viral envelope protein gp120 induces neuropathic pain and spinal gliosis. J Neuroimmunol 2001;116:29-39.
- Zheng W, Ouyang H, Zheng X, Liu S, Mata M, Fink DJ, Hao S. Glial TNFalpha in the spinal cord regulates neuropathic pain induced by HIV gp120 application in rats. Mol Pain 2011;7:40.
- Joseph EK, Chen X, Khasar SG, Levine JD. Novel mechanism of enhanced nociception in a model of AIDS therapy-induced painful peripheral neuropathy in the rat. Pain 2004;107:147-58.
- Zheng X, Ouyang H, Liu S, Mata M, Fink DJ, Hao S. TNFalpha is involved in neuropathic pain induced by nucleoside reverse transcriptase inhibitor in rats. Brain Behav Immun 2011;25:1668-76.
- Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med 2000;29:323-33.
- Crisp T, Minus TO, Coleman ML, Giles JR, Cibula C, Finnerty EP. Aging, peripheral nerve injury and nociception: effects of the antioxidant 16-desmethyltirilazad. Behav Brain Res 2006;166:159-65.
- Khalil Z, Liu T, Helme RD. Free radicals contribute to the reduction in peripheral vascular responses and the maintenance of thermal hyperalgesia in rats with chronic constriction injury. Pain 1999;79:31-7.
- Liu D, Liu J, Sun D, Wen J. The time course of hydroxyl radical formation following spinal cord injury: the possible role of the iron-catalyzed Haber-Weiss reaction. J Neurotrauma 2004;21:805-16.
- Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain 2004;111:116-24.
- Mao YF, Yan N, Xu H, Sun JH, Xiong YC, Deng XM. Edaravone, a free radical scavenger, is effective on neuropathic pain in rats. Brain Res 2009;1248:68-75.
- Schwartz ES, Kim HY, Wang J, Lee I, Klann E, Chung JM, Chung K. Persistent pain is dependent on spinal mitochondrial antioxidant levels. The Journal of neuroscience : the official journal of the Society for Neuroscience 2009;29:159-68.
- Kim HY, Chung JM, Chung K. Increased production of mitochondrial superoxide in the spinal cord induces pain behaviors in mice: The effect of mitochondrial electron transport complex inhibitors. Neurosci Lett 2008;447:87-91.
- Mollace V, Nottet HS, Clayette P, Turco MC, Muscoli C, Salvemini D, Perno CF. Oxidative stress and neuroAIDS: triggers, modulators and novel antioxidants. Trends in neurosciences 2001;24:411-6.
- Yamaguchi T, Katoh I, Kurata S. Azidothymidine causes functional and structural destruction of mitochondria, glutathione deficiency and HIV-1 promoter sensitization. Eur J Biochem 2002;269:2782-8.
- Perl A, Banki K. Genetic and metabolic control of the mitochondrial transmembrane potential and reactive oxygen intermediate production in HIV disease. Antioxid Redox Signal 2000;2:551-73.
- Lewis W, Day BJ, Copeland WC. Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nat Rev Drug Discov 2003;2:812-22.
- Holguin A, O'Connor KA, Biedenkapp J, Campisi J, Wieseler-Frank J, Milligan ED, Hansen MK, Spataro L, Maksimova E, Bravmann C, Martin D, Fleshner M, Maier SF, Watkins LR. HIV-1 gp120 stimulates proinflammatory cytokine-mediated pain facilitation via activation of nitric oxide synthase-I (nNOS). Pain 2004;110:517-30.
- Zheng W, Zheng X, Liu S, Hao S. ROS in the spinal cord is involved in the neuropathic pain induced by antiretroviral drug in rats Society for Neuroscience annual meeting. Washington, DC, 2011.
- Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev 2009;89:121-45.
- Huber R, Pietsch D, Panterodt T, Brand K. Regulation of C/EBPbeta and resulting functions in cells of the monocytic lineage. Cell Signal 2012;24:1287-96.
- Fields J, Gardner-Mercer J, Borgmann K, Clark I, Ghorpade A. CCAAT/enhancer binding protein beta expression is increased in the brain during HIV-1-infection and contributes to regulation of astrocyte tissue inhibitor of metalloproteinase-1. J Neurochem 2011;118:93-104.
- Kanao M, Kanda H, Huang W, Liu S, Yi H, Candiotti KA, Lubarsky DA, Levitt RC, Hao S. Gene Transfer of Glutamic Acid Decarboxylase 67 by Herpes Simplex Virus Vectors Suppresses Neuropathic Pain Induced by Human Immunodeficiency Virus gp120 Combined with ddC in Rats. Anesth Analg 2015;120:1394-404.
- Fink DJ, DeLuca NA, Goins WF, Glorioso JC. Gene transfer to neurons using herpes simplex virus-based vectors. Annu Rev Neurosci 1996;19:265-87.
- Manservigi R, Argnani R, Marconi P. HSV Recombinant Vectors for Gene Therapy. Open Virol J 2010;4:123-56.
- DeLuca NA, McCarthy AM, Schaffer PA. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol 1985;56:558-70.
- Mata M, Hao S, Fink DJ. Applications of gene therapy to the treatment of chronic pain. Curr Gene Ther 2008;8:42-8.
- Glorioso JC, Fink DJ. Herpes vector-mediated gene transfer in the treatment of chronic pain. Mol Ther 2009;17:13-8.
- Simonato M, Bennett J, Boulis NM, Castro MG, Fink DJ, Goins WF, Gray SJ, Lowenstein PR, Vandenberghe LH, Wilson TJ, Wolfe JH, Glorioso JC. Progress in gene therapy for neurological disorders. Nat Rev Neurol 2013;9:277-91.
- Wilson SP, Yeomans DC, Bender MA, Lu Y, Goins WF, Glorioso JC. Antihyperalgesic effects of infection with a preproenkephalin-encoding herpes virus. Proceedings of the National Academy of Sciences of the United States of America 1999;96:3211-6.
- Goss JR, Mata M, Goins WF, Wu HH, Glorioso JC, Fink DJ. Antinociceptive effect of a genomic herpes simplex virus-based vector expressing human proenkephalin in rat dorsal root ganglion. Gene Ther 2001;8:551-6.
- Braz J, Beaufour C, Coutaux A, Epstein AL, Cesselin F, Hamon M, Pohl M. Therapeutic efficacy in experimental polyarthritis of viral-driven enkephalin overproduction in sensory neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 2001;21:7881-8.
- Hao S, Mata M, Glorioso JC, Fink DJ. Gene transfer to interfere with TNFalpha signaling in neuropathic pain. Gene Ther 2007;14:1010-6.
- Hao S, Mata M, Glorioso JC, Fink DJ. HSV-mediated expression of interleukin-4 in dorsal root ganglion neurons reduces neuropathic pain. Mol Pain 2006;2:6.
- Zhou Z, Peng X, Hao S, Fink DJ, Mata M. HSV-mediated transfer of interleukin-10 reduces inflammatory pain through modulation of membrane tumor necrosis factor alpha in spinal cord microglia. Gene Ther 2008;15:183-90.
- Hao S, Mata M, Goins W, Glorioso JC, Fink DJ. Transgene-mediated enkephalin release enhances the effect of morphine and evades tolerance to produce a sustained antiallodynic effect in neuropathic pain. Pain 2003;102:135-42.
- Kanda H, Kanao M, Liu S, Yi H, Iida T, Levitt RC, Candiotti KA, Lubarsky DA, Hao S. HSV vector-mediated GAD67 suppresses neuropathic pain induced by perineural HIV gp120 in rats through inhibition of ROS and Wnt5a. Gene Ther 2016;23:340-8.
- Majima T, Funahashi Y, Takai S, Goins WF, Gotoh M, Tyagi P, Glorioso JC, Yoshimura N. Herpes Simplex Virus Vector-Mediated Gene Delivery of Poreless TRPV1 Channels Reduces Bladder Overactivity and Nociception in Rats. Human gene therapy 2015;26:734-42.
- Oguchi T, Funahashi Y, Yokoyama H, Nishizawa O, Goins WF, Goss JR, Glorioso JC, Yoshimura N. Effect of herpes simplex virus vector-mediated interleukin-4 gene therapy on bladder overactivity and nociception. Gene Ther 2013;20:194-200.
- Wolfe D, Mata M, Fink DJ. A human trial of HSV-mediated gene transfer for the treatment of chronic pain. Gene Ther 2009;16:455-60.
- Fink DJ, Wechuck J, Mata M, Glorioso JC, Goss J, Krisky D, Wolfe D. Gene therapy for pain: results of a phase I clinical trial. Ann Neurol 2011;70:207-12.
- Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther 2010;17:295-304.
- Guedon JM, Wu S, Zheng X, Churchill CC, Glorioso JC, Liu CH, Liu S, Vulchanova L, Bekker A, Tao YX, Kinchington PR, Goins WF, Fairbanks CA, Hao S. Current gene therapy using viral vectors for chronic pain. Mol Pain 2015;11:27.
- Hao S, Liu S, Zheng X, Zheng W, Ouyang H, Mata M, Fink DJ. The role of TNFalpha in the periaqueductal gray during naloxone-precipitated morphine withdrawal in rats. Neuropsychopharmacology 2011;36:664-76.
- Huang W, Zheng W, Liu S, Zeng W, Levitt RC, Candiotti KA, Lubarsky DA, Hao S. HSV-mediated p55TNFSR reduces neuropathic pain induced by HIV gp120 in rats through CXCR4 activity. Gene Ther 2014;21:328-36.
- Huang W, Zheng W, Ouyang H, Yi H, Liu S, Zeng W, Levitt RC, Candiotti KA, Lubarsky DA, Hao S. Mechanical allodynia induced by nucleoside reverse transcriptase inhibitor is suppressed by p55TNFSR mediated by herpes simplex virus vector through the SDF1alpha/CXCR4 system in rats. Anesth Analg 2014;118:671-80.
- Zheng W, Huang W, Liu S, Levitt RC, Candiotti KA, Lubarsky DA, Hao S. IL-10 mediated by herpes simplex virus vector reduces neuropathic pain induced by HIV gp120 combined with ddC in rats. Mol Pain 2014;10:49.
- Iida T, Yi H, Liu S, Huang W, Kanda H, Lubarsky DA, Hao S. Spinal CPEB-mtROS-CBP signaling pathway contributes to perineural HIV gp120 with ddC-related neuropathic pain in rats. Experimental neurology 2016;281:17-27.
- Kanda H, Liu S, Iida T, Yi H, Huang W, Levitt R, Lubarsky DA, Candiotti KA, Hao S. Inhibition of mitochondrial fission protein reduced mechanical allodynia and suppressed spinal mitochondrial superoxide induced by perineural HIV gp120 in rats. Anesth Analg 2016;122:264-72.
- Kim HY, Lee KY, Lu Y, Wang J, Cui L, Kim SJ, Chung JM, Chung K. Mitochondrial Ca2+ uptake is essential for synaptic plasticity in pain. The Journal of neuroscience : the official journal of the Society for Neuroscience 2011;31:12982-91.
- Schwartz ES, Lee I, Chung K, Chung JM. Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain 2008;138:514-24.
- Ferrari LF, Bogen O, Levine JD. Role of nociceptor alphaCaMKII in transition from acute to chronic pain (hyperalgesic priming) in male and female rats. The Journal of neuroscience : the official journal of the Society for Neuroscience 2013;33:11002-11.
- Ohno M, Frankland PW, Silva AJ. A pharmacogenetic inducible approach to the study of NMDA/alphaCaMKII signaling in synaptic plasticity. Curr Biol 2002;12:654-6.
- Minami T, Matsumura S, Mabuchi T, Kobayashi T, Sugimoto Y, Ushikubi F, Ichikawa A, Narumiya S, Ito S. Functional evidence for interaction between prostaglandin EP3 and kappa-opioid receptor pathways in tactile pain induced by human immunodeficiency virus type-1 (HIV-1) glycoprotein gp120. Neuropharmacology 2003;45:96-105.
- Sanna MD, Peroni D, Quattrone A, Ghelardini C, Galeotti N. Spinal RyR2 pathway regulated by the RNA-binding protein HuD induces pain hypersensitivity in antiretroviral neuropathy. Experimental neurology 2015;267:53-63.
- Kann O, Kovacs R. Mitochondria and neuronal activity. Am J Physiol Cell Physiol 2007;292:C641-57.
- Douda DN, Khan MA, Grasemann H, Palaniyar N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proceedings of the National Academy of Sciences of the United States of America 2015;112:2817-22.
- Korbecki J, Baranowska-Bosiacka I, Gutowska I, Chlubek D. The effect of reactive oxygen species on the synthesis of prostanoids from arachidonic acid. J Physiol Pharmacol 2013;64:409-21.
- Gu X, Bo J, Zhang W, Sun X, Zhang J, Yang Y, Ma Z. Intrathecal administration of cyclic AMP response element-binding protein-antisense oligonucleotide attenuates neuropathic pain after peripheral nerve injury and decreases the expression of N-methyl-D-aspartic receptors in mice. Oncol Rep 2013;30:391-8.
- Athos J, Impey S, Pineda VV, Chen X, Storm DR. Hippocampal CRE-mediated gene expression is required for contextual memory formation. Nature neuroscience 2002;5:1119-20.
- Zhang JW, Klemm DJ, Vinson C, Lane MD. Role of CREB in transcriptional regulation of CCAAT/enhancer-binding protein beta gene during adipogenesis. The Journal of biological chemistry 2004;279:4471-8.